Introduction
Skin cancer is the most common type of cancer in the white population [1], and its incidence is rising rapidly in America and Europe. Cutaneous Squamous Cell Carcinoma (cSCC) is a highly prevalent skin cancer [2]. Previous studies have suggested that the development of cSCC may be attributed to long term ultraviolet exposure [3], chemical carcinogens contact [4], receiving immunosuppressive therapy in patients [5,6], which are always accompanied with various levels of immunosuppression. Therefore, enhancement of anti-tumor immunity has been an attractive strategy for intervention of patients with skin cancer.
There are numerous immunological strategies that can stimulate antitumor immunity [7,8], including tumor vaccination, cytokine-mediated immunotherapy, adoptive cellular immunotherapy, and others. Of particular interest is that transplantation with allogeneic cells becomes a promising modality for cancer therapy. A previous study has shown that induction of bone marrow chimeras reduced the size of mammary adenocarcinoma significantly in mice [9]. Furthermore, transplantation with allogeneic hematopoietic stem cells or transfusion of allogeneic lymphocytes effectively eliminated leukaemia and solid tumor [10,11], including renal cell carcinoma, breast cancer, lung cancer [12-14], and others. However, these therapeutic strategies usually have severe adverse effect and may develop Graft-Versus-Host Disease (GVHD) [15,16].
Interestingly, vaccination with allogeneic or syngeneic melanoma cells has been demonstrated to induce Graft-Versus-Tumor (GVT) effect, but does not cause severe adverse effect [17]. Furthermore, intra-tumoral injection with fusogenic membrane glycoproteins from allogeneic tumor cells results in obvious tumor regression [18]. However, injection with CD80 over-expressing allogeneic breast cancer cells did not achieve significant tumor regression [19]. Accordingly, transplantation with MHC-mismatched tumor can induce GVT responses, which may serve as a new anti-tumor therapy. First, tumor cells are usually tolerant to host immunity, which can be broken-down by allogeneic responses [20]. Second, the GVT response is accompanied by allograft rejection, which can inhibit the development of the cancer [21]. In addition, allogeneic Tumor Infiltrating Lymphocytes (TILs) can mediate antitumor response in the non-allosensitized host [22]. Hence, we employed a transplantation model using highly immunogenic skin tumor as allogeneic tumor tissue to assess the GVT effect in mice.
The levels of serum cytokines are valid biomarkers to predict and reflect the alloreactivity [23] and they can be analyzed by Cytometric Bead Array (CBA) technique [24]. Indeed, high levels of IL-2 and IL-4 are detected at the early stage of T-cell activation and during acute rejection. The levels of serum IL-2 are correlated with the outcome of transplanted grafts, and their changes can be used to predict the risk of rejection [23]. Furthermore, elevated levels of serum IL-6 are associated with graft rejection [25]. IL-6 is required for the generation of Th17 cells [26], which mainly produce IL-17A and mediate rejection of allograft rejection [27]. Accordingly, the levels of serum cytokines may be appropriate biomarkers to assess the GVT response.
In this study, we induced cSCC by sequentially exposing to 7, 12-dimethylbenz [a] anthracene (DMBA) and 12-O-tetradecanoyl-phorbol-13-acetate (TPA) in FVB/N mice and ICR mice, and examined the effect of transplantation with allogeneic skin tumors from FVB/N mice to ICR mice with early stage of cSCC. This study was aimed to evaluate safety and therapeutic effect of allogeneic tumor tissue transplantation.
Material and Methods
Animals
Female ICR mice and FVB/N mice at 5-6 weeks of age were purchased from the Slac Laboratory Animal Center (Shanghai, China) and housed in a specific pathogen-free facility of Laboratory Animal Center, Kunming Medical University. They were fed with a standard chow and water ad libitum, and kept on a 12:12 h light-dark cycle at 24 ± 2ºC with 50% ± 10% relative humidity. The animal experimental protocols were approved by the Animal Research and Care Committee of Kunming Medical University, China.
Induction of the skin tumors
The mouse skin tumors were induced by a protocol of two-stage skin carcinogenesis using DMBA as an initiator and TPA as a promoter, as described previously [28,29]. Briefly, individual ICR and FVB/N mice were tropically exposed to 0.025% DMBA in acetone (100 µl/ 3 cm2, Sigma-Aldrich, St. Louis, MO, USA) once on the shaved and depilated dorsal skin. One week later, the same skin area of individual mice were painted with 5 μg TPA (Sigma-Aldrich, St. Louis, MO, USA) in acetone twice within one week. The mice were monitored for the appearance of solid tumors. Individual mice with an induced tumor of 1 mm or greater in a diameter for > 2 weeks were counted and used for the following experiments.
Tumor scoring
Following induction, the mice were monitored for the development of tumors weekly. The numbers of typical papillomas (well-demarcated papules without erosion or ulceration), or carcinomas (poorly demarcated papules with erosion or ulceration) were counted, according to Girardi’s method [30]. Tumor sizes were divided into four grades as below: > 1 mm2, ≥ 4 mm2, ≥ 9 mm2, ≥ 16 mm2.
Body condition scoring
The life quality of individual tumor bearing mice was determined by Body Conditioning Score (BCS) as described previously [31] using the following scores: 1. Muscle wasting is advanced, fat deposits are gone, and bones are very prominent; 2. The mouse is becoming thin and bones are prominent; 3. The mouse is in optimal condition. Bones are palpable but not prominent; 4. The mouse is well-fleshed, and bones are barely felt; 5. The mouse is obese, and bones cannot be felt at all.
Cell counts
To estimate the numbers of tumor cells for transplantation, the resected tumors from FVB/N mice were cut into small pieces, and different amounts of tumor tissues (0.015, 0.03 or 0.045 g) were digested with 0.1% collagenase, 75 U/ml of deoxyribonuclease I, 0.2% hyaluronidase in PBS at 37°C for 3 hours. The resulting cell suspension was washed and the numbers of tumor cells were counted in a blinded manner.
Histopathological analysis
When ulcerative lesions appeared, the tumors were subjected to biopsy. The biopsied tissue samples were fixed in 10% neutral buffered formaldehyde, and embedded in paraffin. The tissue sections (4 µm) were stained with Haematoxylin and Eosin (HE) and examined in a blinded manner as described previously with minor modification [32]. Tumors with papillomatous structure, mild cytologic atypia of keratinocytes, active mitosis limited in the basal and lower epidermis were classified as slight atypical hyperplasia. Tumors with similar architecture, moderate atypical hyperplasia of keratinocytes, and active mitosis over the lower epidermis were classified as moderate atypical papillomas. Tumors with cytologic atypia of keratinocytes, and active mitosis spreading over the entire epidermis were termed severe atypical hyperplasia and SCC in situ; and tumors with isolated atypical keratinocytes over the epidermal-dermal junction were considered as local invasion.
Allogeneic tumor tissue transplantation
To assess the safety of transplantation with allogeneic cutaneous tumor tissue, healthy ICR mice were transplanted subcutaneously (near the inducible skin tumors) with 0.045, 0.03 or 0.015 g tumor tissues from FVB/N mice as the high, medium or low dose group (n = 6 per group), respectively. Their Body Weights (BW), BCS and the volumes of transplanted tissue masses were examined every other day up to 50 days post transplantation and calculated, according to the following formula: ½ (length × width2). Their Body Weights (BW), BCS and the volumes of transplanted tissue masses were examined every other day.
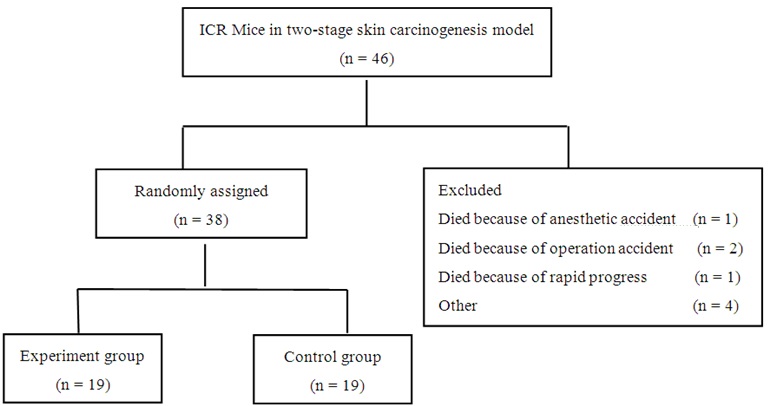
To examine the efficacy of allogeneic tumor transplantation, ICR mice were induced for skin tumors and when most induced tumors started to show visible erosion or ulceration, the mice were randomly divided into two groups. The experiment group was transplanted subcutaneously with 0.3 g skin tumors from FVB/N mice three times with an interval of 1-2 weeks; the control mice received the sham surgery at each time point (n = 19 per group). The characteristics of both groups of mice before the allogeneic tumor transplantation were evaluated (Table 1). Following transplanted with allogeneic skin tumors, BW, BCS, tumor volumes in situ, metastasis tumors, overall survival and serum cytokines were measured longitudinally.
PET/CT imaging
The potential tumor metastasis was examined by PET-CT, which was tested before and 24 days after transplantation using a combined PET/CT scanner (Biograph 16, Siemens, Erlangen, Germany), as described previously with minor modification [33]. Briefly, the mice were fasted for 12 hours, and anesthetized intraperitoneally with pentobarbital sodium (40 mg/kg). Subsequently, individual mice were injected intravenously with approximately 7.4 MBq (200 μCi)/200 μL fluorin-18-2-fluoro-2-deoxy-d-glucose (18F-FDG) and scanned for an increased rate of aerobic glycolysis at 45 minutes post injection with probe. Two-dimensional PET, CT, and merged PET/CT images were reconstructed and data were analyzed using the AMIDE software.
Cytometric bead array
The levels of serum IL-2, IL-4, IL-6, IFN-, TNF, IL-17A, and IL-10 in individual mice were measured before and one and two weeks after the first transplantation by Cytometric Bead Array (CBA) using a mouse Th1/Th2/Th17 cytokines kit (BD Biosciences, San Jose, CA, USA) on the FACS Calibur flow cytometer. The data were analyzed using the BD Biosciences CBA software.
Statistics
Continual Data are expressed as means ± standard deviations, medians and inter-quartile ranges, dependent on their distribution. Categorical data are expressed as the real counts and percentages. The difference between groups over a period was analyzed by repeated measurement using mixed effect model or analyzed by Student t test, Wilcoxon rank sum test or Wilcoxon matched-pairs signed rank sum test, or the Fisher’s exact test. Survival curves were plotted using the Kaplan-Meier method and were compared by the log-rank test. Statistical analyses were performed using SPSS 19.0 statistic software (SPSS Inc., Chicago, IL, USA). A P value of < 0.05 was considered statistically significant.
Results
Chemical induction of skin tumors in FVB/N mice
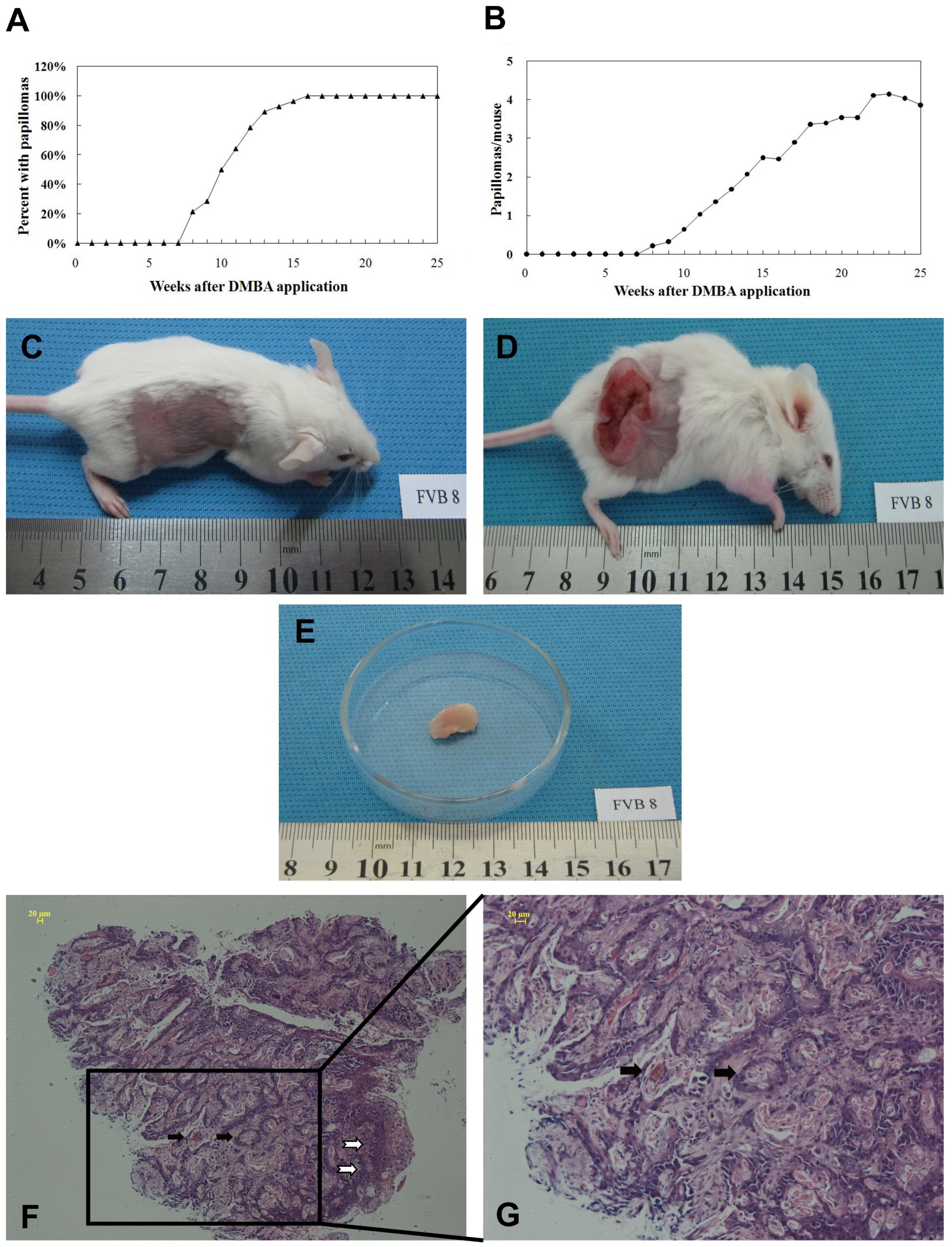
To induce skin tumors, FVB/N mice were sequentially painted with DMBA and TPA and the development of tumors were monitored. We found that tumors began to occur at 8 weeks post induction and all mice developed the skin tumor at 16 weeks post induction (Figure 1A). Quantitative analysis indicated that there were 3.86 ± 2.01 papillomas per mouse at 25 weeks post induction (Figure 1A and B). Histologically, chemical exposure induced cutaneous papillomas always showed visible erosion or ulceration (Figure 1C-G), which included many neoplastic mitotic cells and atypical epithelium as well as an asymmetric outline, the hallmarks of early stage of cSCC.
Results
Chemical induction of skin tumors in FVB/N mice
To induce skin tumors, FVB/N mice were sequentially painted with DMBA and TPA and the development of tumors were monitored. We found that tumors began to occur at 8 weeks post induction and all mice developed the skin tumor at 16 weeks post induction (Figure 1A). Quantitative analysis indicated that there were 3.86 ± 2.01 papillomas per mouse at 25 weeks post induction (Figure 1A and B). Histologically, chemical exposure induced cutaneous papillomas always showed visible erosion or ulceration (Figure 1C-G), which included many neoplastic mitotic cells and atypical epithelium as well as an asymmetric outline, the hallmarks of early stage of cSCC.
Allogeneic tumor tissue transplantation from FVB/N mice to ICR mice got short-term graft-versus-tumor effects but not long-term efficacy
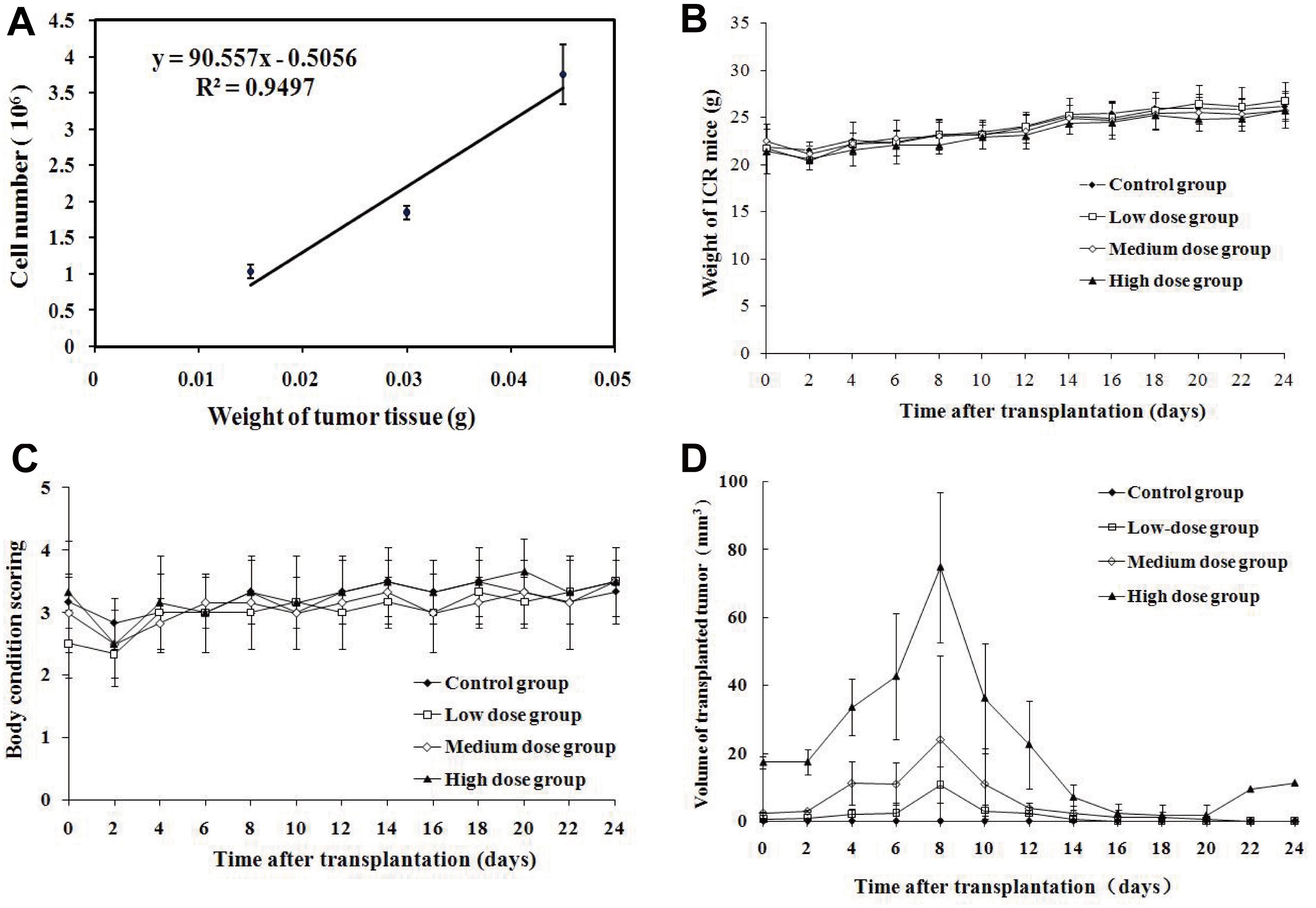
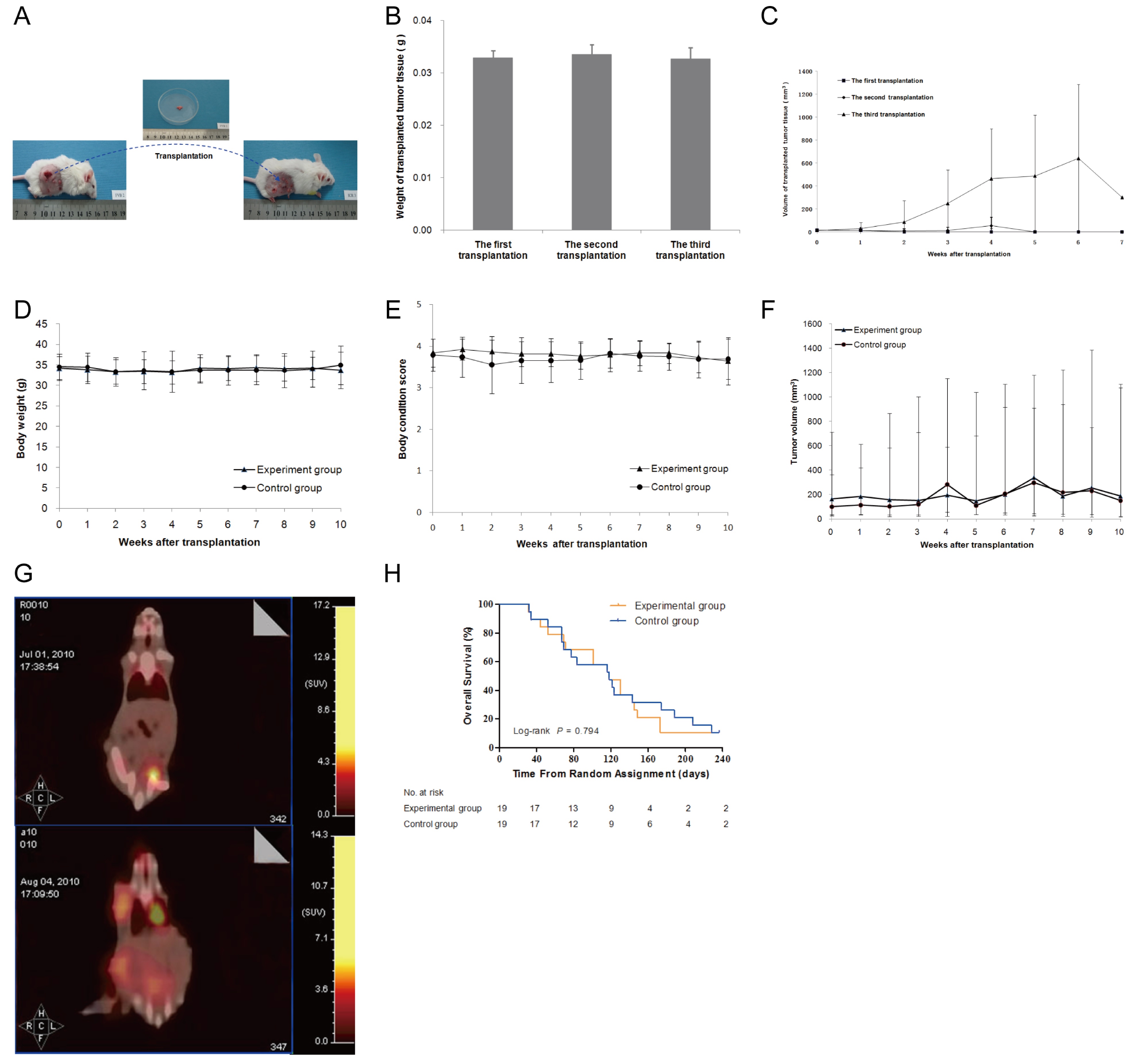
To test the safety of allogeneic tumor transplantation, we first assessed quantitatively the numbers of tumor cells in skin tumor tissues and found that the numbers of tumor cells were correlated linearly with the tumor weights (Figure 2A). Furthermore, we transplanted different doses of skin tumor tissues from FVB/N mice to healthy ICR mice and we found that transplantation with any of the doses of skin tumor tissues did not affect BW and BCS (p > 0.05, figure 2B and C). Measurements of tumors revealed that the transplanted tumor grew rapid and the tumor volumes peaked at 8 days post inoculation and gradually declined (Figure 2D). From d0 to d14, the transplanted tumor sizes in the high dose group were bigger than the other two groups (p < 0.05), and those in the medium dose group were bigger than the low dose group (p < 0.05). While most transplanted tumors disappeared after 20 days, only one tumor from the high dose group grew back after transient shrinking down. These data suggest that transplantation with no more than 0.03 g tumor tissues was safe in mice.
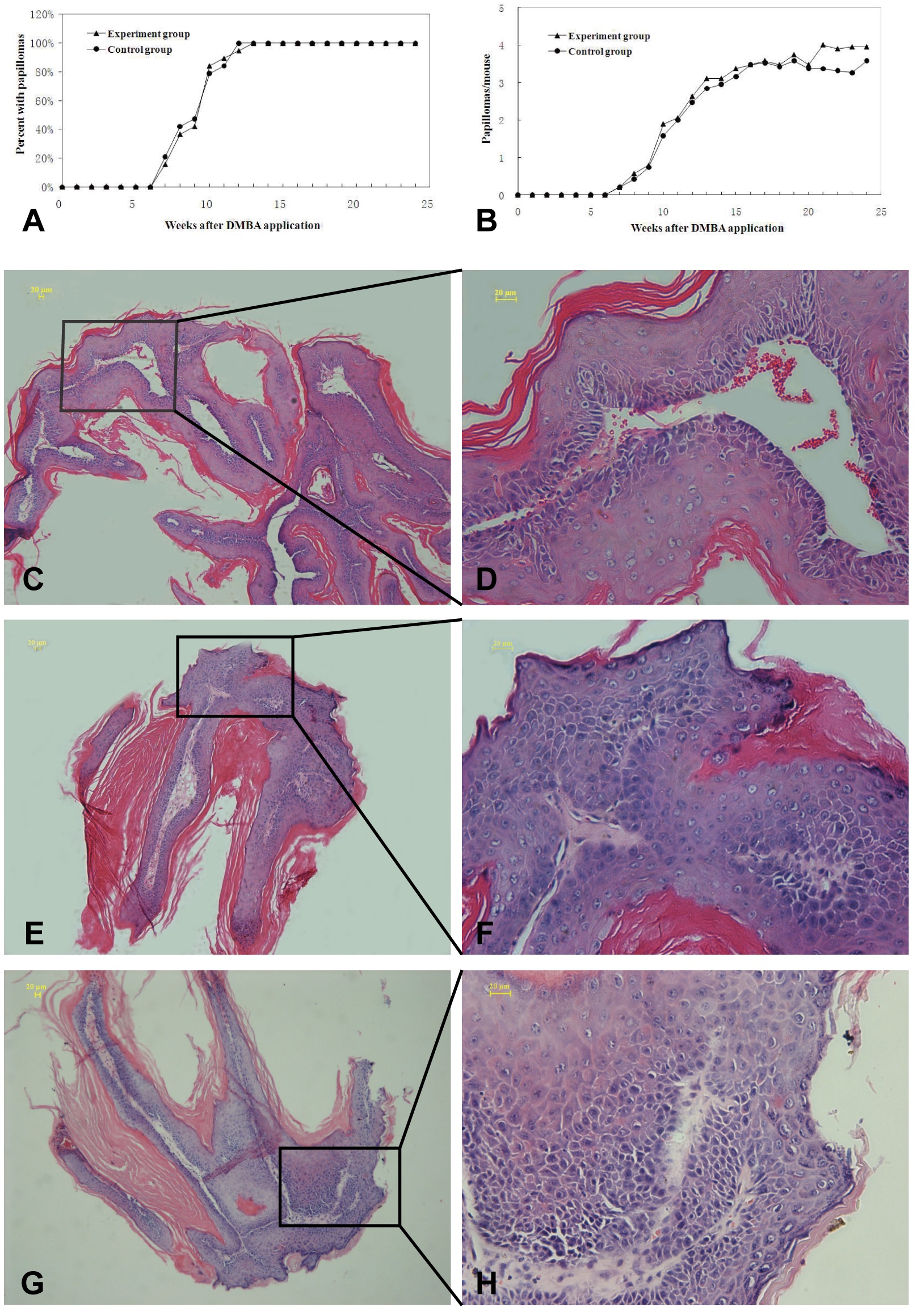
Next, we tested whether transplantation with allogeneic skin tumors could inhibit the progression of chemicals-induced skin tumors. ICR mice were chemically induced for the development of skin tumors and randomized into two groups (Table 1), the experimental group and control group (Figure 3). Following induction, all mice developed skin tumors at 12-13 weeks post induction and the mean numbers of papillomas were 3.95 ± 2.32 and 3.58 ± 2.04 per mouse between the experimental and control mice at 24 weeks post induction (Figure 4A and B). Histological analysis revealed that the tumors with visible erosion or ulceration in the skin papilloma showed different degrees of atypical hyperplasia (Figure 4C-H).
(A,B) The growth kinetics of induced papillomas and the average numbers of papillomas per mouse in the experiment group and the control group; (C,D) The representative HE staining shows mild atypical hyperplasia; (E,F) The representative HE staining shows moderate atypical hyperplasia, and (G,H) The representative HE staining shows severe atypical hyperplasia and cutaneous squamous cell carcinoma in situ. The white arrows showed representative histopathological characteristics of the skin tumor. Bar: 20 μm.
Subsequently, the tumor-bearing ICR mice in the experimental group were transplanted subcutaneously with 0.03 g skin tumors from FVB/N mice for three times and the tumor-bearing ICR control mice received the sham surgery (Figure 5A and B). Following transplantation, the tumor transplanted in the first and second time grew slowly and the tumor volumes peaked at 4 weeks post transplantation and gradually disappeared (Figure 5C). Interestingly, the tumors transplanted at the third time grew rapid and peaked at 6 weeks post transplantation. Subsequently, most tumors disappeared except for one mouse with transplanted tumor, which grew continuously and the mouse died on day 173.
Further analyses indicated that similar BW, BCS and tumor volumes were measured in the experimental and control mice (Figure 5D-F). While BCS and tumor sizes in the experiment group were superior to those in the control group in the first 3 weeks after transplantation, but with no statistical significance. However, PET-CT imaging revealed that there were four experimental mice with obvious metastatic tumors while there were nine control mice with metastatic tumors at 24 days post transplantation (Figure 5G, table 2). Furthermore, there was no significant difference in the survival periods between two groups of mice (Figure 5H). Collectively, these data suggest that transplantation with allogeneic skin tumor inhibited the early growth and metastasis of skin tumors, but did not affect the overall survival of tumor-bearing mice.
Cytokine changes after the allogeneic skin tumor tissue transplantation
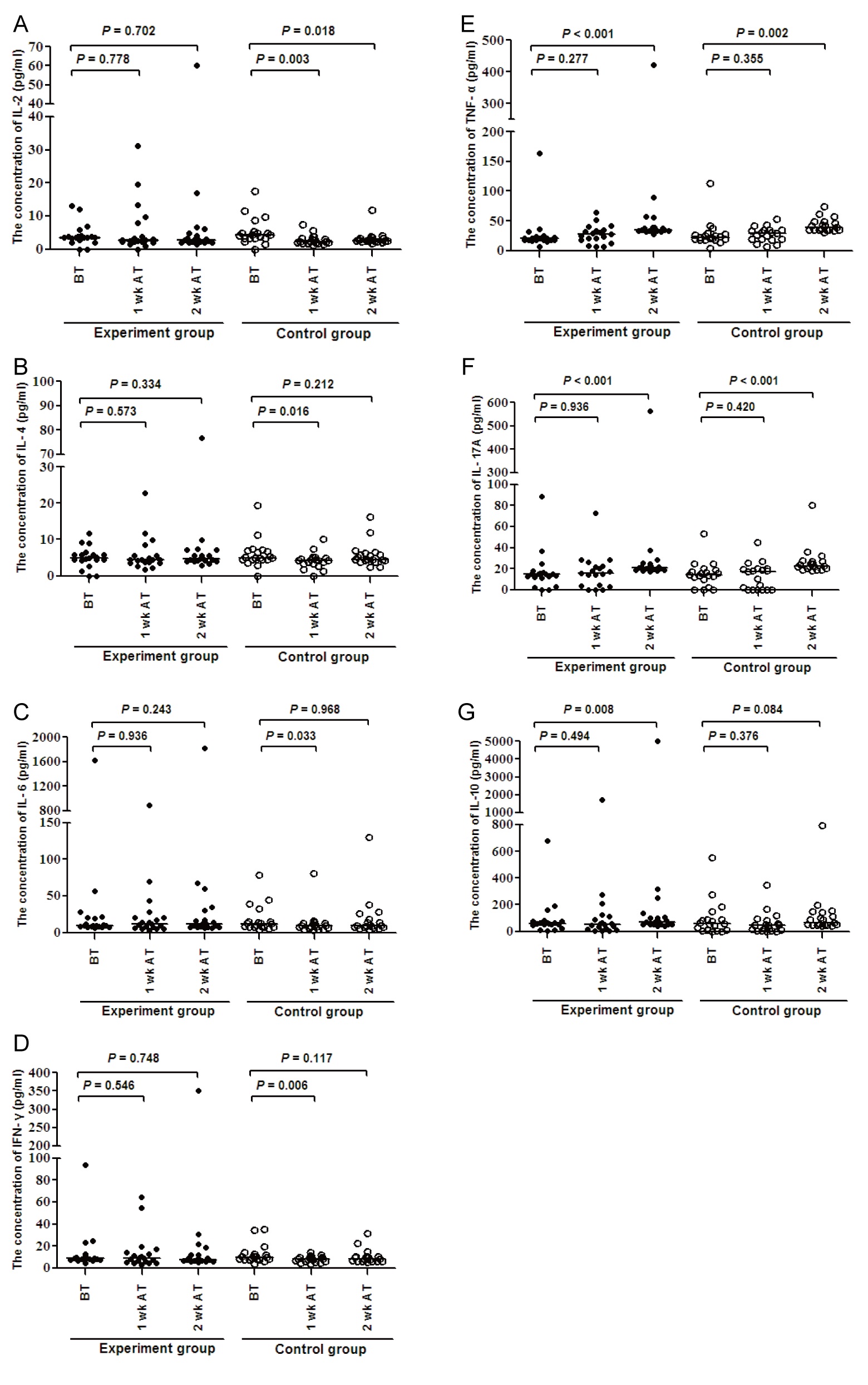
To further understand the effect of transplantation with allogeneic skin tumor, we tested the levels of serum cytokines by CBA. In comparison with that before transplantation, the levels of IL-2, IL-4, IL-6, and IFN- in the control group decreased significantly after one week post transplantation, (P < 0.05, figure 6). In contrast, the levels of IL-2, IL-4, and IFN- in the experiment group only decreased slightly, but IL-6 increased slightly. TNF-α, IL-10 and IL-17A did not have significant change one week post transplantation. Two weeks after the transplantation, the level of IL-2 were still significantly lower (P < 0.05), while IL-2 in the experiment group remained stable. The levels of TNF-α and IL-17A in these two groups increased significantly (P < 0.05). Unexpectedly, the levels of IL-10 in the experiment group increased significantly (P < 0.05), while increased moderately in the control group. IL-4, IL-6, and IFN- did not have significant change two weeks post transplantation. And there was no significant difference between these two groups.
Discussion
To evoke GVT immunity using allogeneic transplantation is an attractive method to treat cancer, many experiments were explored to verify the anticancer effect of GVT. While natural antitumor immunity exists in humans [20], transplantation with ethanol extracts of homologous tumors did not have therapeutic effect [34]. Interestingly, the effectiveness of GVT was observed in the mammary carcinoma and renal-cell carcinoma, which make the allogeneic transplantation get new attention [35,36]. In this study, we employed a protocol of mouse two-stage skin carcinogenesis using DMBA/TPA to induce skin tumors in two different strains of mice to test the effect of transplantation with allogeneic skin tumors on the growth of chemically induced tumors in the recipients.
Previous studies have suggested that tumors growth is associated with immunosuppression [37,38], and high levels of novel antigens can be detected at early stage of chemically induced tumors [37,39]. In our experiment, we found that all mice exposed chemicals developed typical papillomas at 12-13 weeks post induction, similar to that of previous reports [40]. And we chose to transplant with early stage of skin tumors for three times to induce potent GVT responses. We found that the transplanted tumors in the first and second transplantation disappeared after short-term increase, while in the third transplantation; the transplanted tumor in one mouse continually grew until the mouse died later. These data suggest that continual transplantation with the same allogeneic skin tumors may induce immune suppression and tolerance, which promotes the growth of transplanted allogeneic tumors in recipients.
There are many attempts to treat solid tumors using allogeneic tumor cells or gene modification allogeneic tumor cells [17,18]. In our study, we also found that transplantation with allogeneic skin tumors slightly reduced the volumes of endogenous skin tumors in the first 3 weeks and the percentages of mice with metastatic tumors in the recipients for a short period around 24 days post the transplantation, but with no long-term efficacy. These data indicated that transplantation with allogeneic skin tumors could temporarily inhibited the growth and metastasis of endogenous skin tumors in the recipients. Indeed, a previous study has shown that transfused donor WBCs exists for a short period in the patients who received multiple blood transfusions [41] and activated antitumor T lymphocytes maintain in peripheral blood for a short period or even become anergic within 2 weeks following transition [42]. Apparently, transplantation with allogeneic skin tumors induced certain antitumor responses, which temporarily inhibited the growth and metastasis of endogenous skin tumor in the recipients.
Compared with skin tumor and squamous cells carcinoma, there are more attempts to treat melanoma using allogeneic melanoma cell vaccine or gene-transfected melanoma cell vaccine. In the murine melanoma models, both live and irradiated allogeneic tumor cells vaccination could induce an anti-tumour effect using only one immunization and with no boost or adjuvant [17]. Labarthe MC et al., found that allogeneic B16-F10 melanoma cells could generate cytotoxic T lymphocytes and an anti-tumour effect by cross-reactive immunity, and cells labeled with fluorescent dye remained at the injection site for only 9 days which means the potent immune responses are temporary [43]. Agree with Labarthe MC, we also found that transplantation with allogeneic tumor tissue could induce a short-term anti-tumor response in skin tumors.
The changes in the levels of serum cytokines may represent the degrees of acute and chronic allogeneic rejection response [23]. In this study, we found significantly reduced levels of IL-2, IL-4, IL-6 and IFN-γ in the control mice, but not the recipients at one week post transplantation; lower level of IL-2 in the control group, higher levels of IL-17 and TNF-α in the both groups of mice and significantly higher levels of serum IL-10 in the recipients at two weeks post transplantation, similar to that of previous reports [23,44]. Our results showed that IL-2 decreased significantly in the control group, while kept stable in the experiment group, which may be correlated with the short-term efficacy. TNF-α and IL-17 increased with the growth of the tumor in the two groups, supporting the notion that they may be tumor promoting factors, linking inflammation and cancer [45,46], although high levels of TNF-α can inhibit tumor development [47], and IL-17 may take part in reject reaction [26]. We also detected higher levels of serum IL-10 in the recipients. Although IL-10 has been considered as inhibitory cytokine associated with regulatory T and B cell responses, IL-10 can also promote CD8+ T cell responses [48]. IL-10 may be involved in the maintenance of CTL in the recipients. Therefore, the changes in the levels of serum cytokines may be associated with temporary inhibition on the growth and metastasis of endogenous skin tumors in the recipients.
In summary, our data indicated that transplantation with no more than 0.03 g allogeneic skin tumors to health mice was safe, and transplantation with allogeneic skin tumors to tumor-bearing mice could temporarily inhibited the growth and metastasis of endogenous skin tumors in the recipients, which may be correlated with the graft-versus-tumor reponse media by the alteration of IL-2. Our findings suggest that transplantation with allogeneic skin tumors may have temporary anti-tumor effect, while there are still much work needed to do for magnifying the GVT response and improving the long-term efficacy.
Conflicts of Interest/Financial Disclosures
This study was supported in part by grants from National High Technology Research and Development 863 Program of China (#2012AA02A201), National Key Technology Research and Development Program of The 12th Five-Year Plan of China (#2011ZX09102-001-29), National Natural Science Foundation of China (#81360322, #81060185, #81260307, and #81470005), National Clinical Key Specialty Construction Projects of Oncology of National Health and Family Planning Commission of China (Awarded to Tumor Hospital of Yunnan Province: 2013-2014), Leading Talent Program of Health Systems of Yunnan Province (#L-201213), the Yunnan Provincial General Program for Applied Basic Research (#2011FB209 and #2012FB069).All the authors have no financial conflicts of interest with regard to this work.