
Effect of Glucose on Retinal Endothelial Cell Viability and VEGF Secretion
*Corresponding Author(s):
Andrew T TsinDepartment Of Biology, The University Of Texas At San Antonio, Texas, United States
Tel:+1 2104585480,
Email:andrew.tsin@utsa.edu
Abstract
Previous studies have shown that in diabetic patients, there is an increase of retinal capillaries associated with the development of diabetic retinopathy in the eye. The objective of current study is to investigate the effect of glucose on retinal endothelial cell viability and VEGF secretion. 20,000 cells per well were treated without glucose or with 5.5mM (euglycemic), 18.5mM and 30mM (hyperglycemic) glucose for 24 hours. Viable cells were counted using Trypan blue dye exclusion method. ELISA was used to measure VEGF secretion from cells into the cell medium. The number of viable cells incubated with 5.5mM glucose (physiological control) increased by 53.7% after 24 hours. In comparison, cells treated with 18.5mM glucose decreased by 2.8% while cells treated with 30mM glucose decreased by 20% after 24 hours of incubation. Cells without glucose treatment
(0mM control) decreased by 33.3%. In contrast to the decrease of viable cell numbers after treatment with high glucose, there is an increase in VEGF secretion (pg/mL) to the cell medium with increase in glucose concentration from 5.5mM to 0, 18.5, and 30mM. The amount of VEGF secreted per cell also increased with increasing glucose concentrations. Our results show that viability of retinal endothelial cells and VEGF release are highly responsive to changes in glucose concentration. Such glucose-induced changes in retinal endothelial cells may negatively impact the integrity of the microvasculature in the diabetic retina leading to angiogenesis and microaneursyms.
Keywords
INTRODUCTION
Diabetes mellitus is a metabolic disorder characterized by high levels of glucose in the blood (hyperglycemia). Diabetes is highly prevalent in developing countries and is estimated to affect approximately 439 million people by 2030 [1], suggesting that diabetes is a global health problem. This chronic disease has long-term effects on kidneys, nerves, the heart, and other major organs [2,3]. One such complication affects the eye and is known as Diabetic Retinopathy (DR). DR is one of the leading causes for blindness, which affects approximately 93 million people worldwide. The key determinants in development of DR are diabetic duration, glycemic control, and genetics [4,5]. In the United States, 1 out of 12 persons with diabetes mellitus, age 40 and older, has advanced vision threatening retinopathy [6]. Angiogenesis is a significant process in the development of diabetic retinopathy. A strong controller and promoter of angiogenesis, Vascular Endothelial Growth Factor (VEGF) is a specific mitogen that induces new blood vessel formation by targeting growth and differentiation of endothelial cells [7]. In diabetic patients, VEGF is excessively produced in the retina resulting in endothelial cell proliferation, tissue damage, fluid leakage, new blood vessel formation [8], and microaneurysms. The polymorphisms in the VEGF gene are associated with VEGF expression and diabetic retinopathy [9,10]. Previously, we reported high glucose changes cell viability and VEGF secretion in Müller cells, retinal Pericytes, and ARPE 19 cells [11-13]. We hypothesize that the viability of retinal endothelial cells and their secretion of VEGF is significantly impacted by changes in glucose concentration. The objectives of our current study is to compare the effects of high glucose on viability of endothelial cells and their VEGF secretion compared to no-glucose and physiological glucose controls. Our novel findings in Rhesus Monkey retinal endothelial cells may help us establish a model for further research in the diabetic, non-human primate eye.
METHODS
Cell culture
Glucose treatments
Trypan blue dye exclusion method
hVEGF ELISA
Statistical analysis
RESULTS
Concentration-dependent change in cell viability
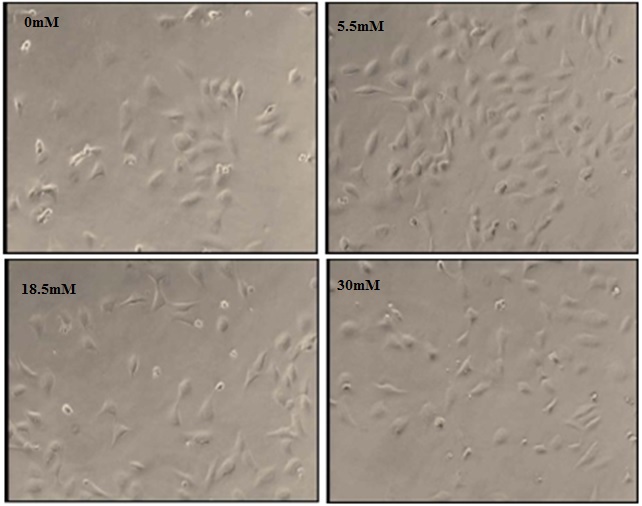
Figure 1: Photomicrographs of RhREC grown in 0, 5.5, 18.5 and 30mM glucose for 24 hours.
Images from cells in 5.5mM show 75% confluence, a significant increase from T0 (60%). In comparison, images from cells in 0mM and 30mM show 45% and 50% confluence, a significant decrease from T0density and a notable decrease in confluence was observed in cells incubated at 18.5mM. All images were taken at 100X magnification.
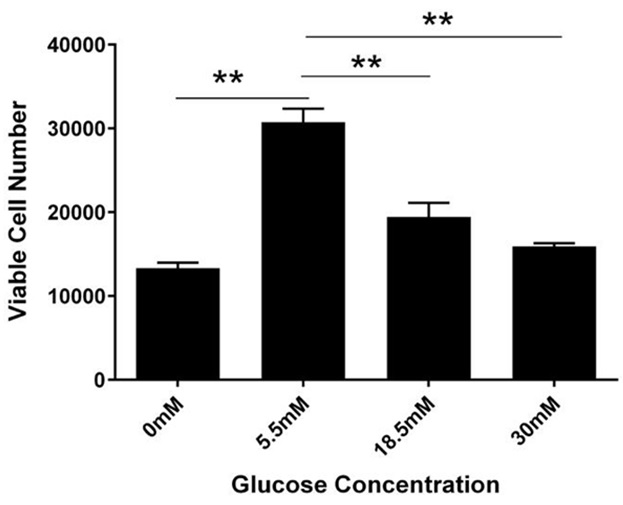
Figure 2: Concentration-dependent change in cell viability.
RhREC were plated at 20,000 per ml in a 24 well plate. After 24 hours of glucose treatment, there were 13,333 cells in 0mM, 30,741 cells in 5.5mM, 19,444 in 18.5mM and 15,926 in 30mM. One-way ANOVA determined the difference between groups was significant (F (3, 7) =50.48, P=0.00004). A Dunnett’s multiple comparison test was performed using 5.5mM as control (**P<0.0001).
Concentration-dependent change in VEGF level in cell media
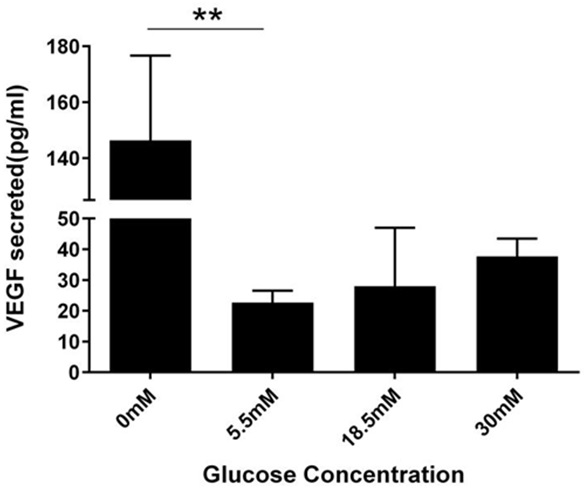
Figure 3: Concentration-dependent change in VEGF secretion per mL.
After 24 hour glucose treatment, VEGF amounts secreted into conditioned medium was measured for by ELISA. Cells in 0mM secreted 146.3pg/ml of VEGF, 22.6pg/mL in 5.5mM, 28pg/mL in 18.5mM, and 37.6pg/mL in 30mM. Results from One way ANOVA were significant (F (3, 7) =10.74, P=0.0052). A Dunnett’s multiple comparison test was performed using 5.5mM as control (**P<0.05).
The VEGF per cell (pg/cell) was calculated by dividing the VEGF levels in cell medium by their respective cell number. After 24 hours treatment, each cell in 5.5mM glucose secreted 0.00073pg of VEGF, which was doubled when the glucose concentration was increased to 18.5mM (0.0015 pg/cell) and tripled when glucose concentration was further increased to 30mM (0.002 pg/cell). A 13-fold increase was observed in cells grown in 0mM glucose (0.01 pg/cell) (Figure 4). One-way ANOVA showed a highly significant glucose effect on VEGF secretion (pg/cell; P≤0.00023).
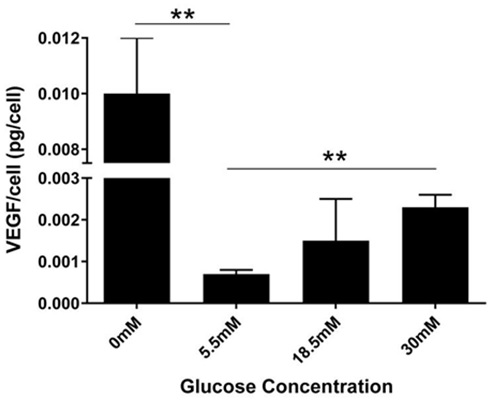
Figure 4: Concentration dependent change in VEGF secretion per cell.
VEGF/cell was calculated by dividing VEGF levels measured in cell medium with respective cell number. After 24 hours, cells treated with 0mM glucose secreted approximately 0.01pg/cell, 0.00073pg/cell in 5.5mM, 0.001pg/cell in 18.5mM, and 0.0023pg/cell in 30mM. Results from one way ANOVA showed significance (F (3, 7) =14.17, P=0.0023). A Dunnett’s multiple comparison test was performed using 5.5mM as control (**P<0.05). T-Test showed significant difference (5% level) in the level of VEGF between the 5.5mM vs the 30mM treatment groups.
DISCUSSION
In the present study, it was observed that changes in RhREC confluence and viability over a 24 hour period was dependent on glucose concentration. Physiological glucose significantly increased cell number but hyper- or hypoglycemic conditions induced a significant decrease in cell number (Figure 2). Our results validate the hypothesis that a change in glucose concentration induces change in the number of RhREC. It is of great interest to point out the 18.5mM glucose treatment did not result in a large change in cell number compared to T0. When related to diabetic conditions in humans, it is possible that uncontrolled hyperglycemic conditions in excess of plasma levels of 18.5mM may result in a large reduction in retinal endothelial cells leading to complications such as angiogenesis and microaneurysms. We have previously reported that treatment of high glucose (18mM, 33mM and 40mM) for a period of 5 days caused significant decreases in cell viability in primary human retinal pericytes [12]. When ARPE 19 cells were treated with high glucose (18mM) for 8 days there were significant increases in cell viability [13]. Retinal Müller cells treated with no glucose for 24 hours resulted in a decrease in cell viability, however high glucose (30mM) increased cell viability [11]. Previous studies on primary human retinal endothelial cells show that high glucose increased cell viability [14,15]. The differences in our findings compared to those published are likely due to inter- species differences (human vs rhesus monkey) as well as the transformation of the rhesus monkey retinal endothelial cells compared to the primary human line reported by our colleagues.
RhREC subjected to treatment with higher glucose concentrations resulted in a significant increase in VEGF levels in the conditioned media (Figure 3). Dunnett’s multiple comparison test suggests a significant change between VEGF levels in RhREC treated with 0mM or 5.5mM as well.
In human synovial fibroblasts, treatment with 33mM glucose for a 24 hour period resulted in an increase in VEGF secretion from the cells into the medium [16]. In earlier reports, high glucose not only induced changes in viable cell number but also increased VEGF secretion in cell medium from human retinal pericytes and ARPE-19 [12,13]. Vellanki et al., 2016 showed that both high and low glucose treatments resulted in an increase in VEGF secretion into cell medium from rat and human müller cells [11]. Primary human retinal endothelial cells show that high glucose stimulates VEGF expression [14,15] and Human Umbilical Vein Endothelial Cells (HUVEC) treated with high glucose (25mM/L) resulted in a decrease in VEGF secretion in cell medium. This contrasting effect to our reports may be due to the difference in cell type and exposure time compared to our rhesus monkey cell line [17].
In the current study, the amount of VEGF secreted per cell increased significantly with changes in glucose concentration from 0, 18.5 or 30mM compared to physiological (5.5mM) conditions (Figure 4). These results are consistent with our previous findings in which increasing glucose concentrations to 18.5mM resulted in an increase in VEGF secretion per cell from both ARPE-19 and human retinal pericytes.
Our study observed that glucose deprived conditions induce a more pro-angiogenic response than high glucose conditions. It is not clear how VEGF affects RhREC proliferation. Further studies are required to determine the interactive effect of glucose, VEGF, RhREC proliferation, and VEGF levels inside the cell. It is possible that glucose may induce apoptosis of RhREC via a molecular mechanism involving TGFβ similar to that reported earlier [18]. Overall, our data clearly suggests that glucose concentration has a significant effect on the viability of RhREC in culture as well as their secretion of VEGF. We plan to extend our current results to include longer time points and assays to better understand the mechanism of viability change as well as the changes in VEGF secretion. Nevertheless, such glucose effects on RhREC number and VEGF release are novel and important as they provide innovative approaches to investigate the cause of angiogenesis in diabetic retinopathy.
ACKNOWLEDGEMENTS
Authors thank the RCMI Program from the National Institutes of Minority Health and Health Disparities (G12MD007591) at the National Institute of Health and the IMARI Program at Oakwood University.
REFERENCES
- Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87: 4-14.
- Shah A, Kanaya AM (2014) Diabetes and associated complications in the South Asian population. Curr Cardiol Rep 16: 476.
- Petrovic D (2010) The role of vascular endothelial growth factor gene as the genetic marker of atherothrombotic disorders and in the gene therapy of coronary artery disease. Cardiovascular & hematological agents in medicinal chemistry 8: 47-54.
- Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, et al. (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35: 556-564.
- Petrovi? D (2013) Candidate genes for proliferative diabetic retinopathy. Biomed Res Int 2013: 540416.
- Kempen JH, O’Colmain BJ, Leske MC, Haffner SM, Klein R, et al. (2004) The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol 122: 552-563.
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, et al. (2004) Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 56: 549-580.
- Tolentino MJ, Miller JW, Gragoudas ES, Jakobiec FA, Flynn E, et al. (1996) Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology 103: 1820-1828.
- Petrovic MG, Korosec P, Kosnik M, Osredkar J, Hawlina M, et al. (2008) Local and genetic determinants of vascular endothelial growth factor expression in advanced proliferative diabetic retinopathy. Mol Vis 14: 1382-1387.
- Awata T, Inoue K, Kurihara S, Ohkubo T, Watanabe M, et al. (2002) A common polymorphism in the 5’-untranslated region of the VEGF gene is associated with diabetic retinopathy in type 2 diabetes. Diabetes 51: 1635-1639.
- Vellanki S, Ferrigno A, Alanis Y, Betts-Obregon BS, Tsin AT (2016) High Glucose and Glucose Deprivation Modulate Müller Cell Viability and VEGF Secretion. Int J Ophthalmol Eye Res 4: 178-183.
- Vidro EK, Gee S, Unda R, Ma JX, Tsin A (2008) Glucose and TGFbeta2 modulate the viability of cultured human retinal pericytes and their VEGF release. Curr Eye Res 33: 984-993.
- Heimsath EG Jr, Unda R, Vidro E, Muniz A, Villazana-Espinoza ET, et al. (2006) ARPE-19 cell growth and cell functions in euglycemic culture media. Curr Eye Res 31: 1073-1080.
- Sun J, Xu Y, Sun S, Sun Y, Wang X (2010) Intermittent high glucose enhances cell proliferation and VEGF expression in retinal endothelial cells: the role of mitochondrial reactive oxygen species. Mol Cell Biochem 343: 27-35.
- Wang D, Wang L, Gu J, Yang H, Liu N, et al. (2014) Scutellarin inhibits high glucose-induced and hypoxia-mimetic agent-induced angiogenic effects in human retinal endothelial cells through reactive oxygen species/hypoxia-inducible factor-1α/vascular endothelial growth factor pathway. J Cardiovasc Pharmacol 64: 218-227.
- Tsai CH, Chiang YC, Chen HT, Huang PH, Hsu HC, et al. (2013) High glucose induces vascular endothelial growth factor production in human synovial fibroblasts through reactive oxygen species generation. Biochim Biophys Acta 1830: 2649-2658.
- Yang Z, Mo X, Gong Q, Pan Q, Yang X, et al. (2008) Critical effect of VEGF in the process of endothelial cell apoptosis induced by high glucose. Apoptosis 13: 1331-1343.
- Mondragon AA, Betts-Obregon BS, Moritz RJ, Parvathaneni K, Navarro MM, et al. (2015) BIGH3 protein and macrophages in retinal endothelial cell apoptosis. Apoptosis 20: 29-37.
Citation: Betts-Obregon BS, Vellanki S, Buikema J, Tsin AT, Wright K (2016) Effect of Glucose on Retinal Endothelial Cell Viability and VEGF Secretion. J Cell Biol Cell Metab 3: 008.
Copyright: © 2016 Brandi S Betts-Obregon, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

