ABSTRACT
Congenitally corrected transposition of the great arteries is a rare heart defect that can be associated with systemic ventricular dysfunction and conduction disturbances. The use of cardiac resynchronization therapy in patients with congenital heart disease is not fully established and achievement of successful pregnancies after implantation of transvenous, biventricular system have never been described, and which resulted in a significant clinical improvement.
We describe a 33-year-old female with congenitally corrected transposition of the great arteries, who achieved six pregnancies and successful vaginal deliveries. The two last pregnancies were achieved after cardiac resynchronization therapy for systemic ventricular dysfunction and complete heart block. A congenital cardiac disease have been identified in only one offspring.
KEYWORDS
Cardiac resynchronization therapy pregnancy; Congenitally corrected transposition of the great arteries
Abbreviations
AV : Atrio-Ventricular
AVB : Atrioventricular Block
ASD : Atrial Septal Defect
ccTCGA : congenitally corrected Transposition of the Great Arteries
CRT : Cardiac Resynchronisation Therapy
CS : Coronary Sinus
LV : Left Ventricle
PLVC : Posterolateral Cardiac Vein
RV : Right Ventricle
RVEF : Right Ventricle Ejection Fraction
Introduction
Congenitally corrected Transposition of the Great Arteries (ccTGA) accounting for less than 1% of congenital heart disease [1]. Most women with ccTGA reach childbearing age. The morphologic Right Ventricle (RV) is not designed to support systemic circulation and particularly increased cardiac output during pregnancy and is predisposed to dysfunction [2-4].
Cardiac Resynchronization Therapy (CRT) is supposed to stabilize if not to improve cardiac function in these patients [5-7].
To our knowledge this is the first case reported of cardiac resynchronization therapy by transvenous access in a patient with congenitally corrected transposition of the great arteries with complete Atrioventricular Block (AVB) and systemic ventricular dysfunction who achieved six successful pregnancies and vaginal deliveries, four before and two after cardiac resynchronization therapy.
Case Report
We report a case of a 33-year-old female with congenitally corrected transposition of the great arteries, who was admitted to our institution because of an exacerbation of heart failure (NYHA class III heart failure symptoms), recurrent dizzy spells and general physical weakness.
At the age of 22, she underwent surgery of an ostium primum Atrial Septal Defect (ASD) closure complicated by a right bundle branch block. Initial postoperative atrioventricular conduction was normal. No anatomical repair with double switch operation was performed. She has also the history of 4 pregnancies and successful vaginal deliveries. The patient remained asymptomatic until the age of 33, when she started experiencing dyspnea, fatigue and dizzy spells.
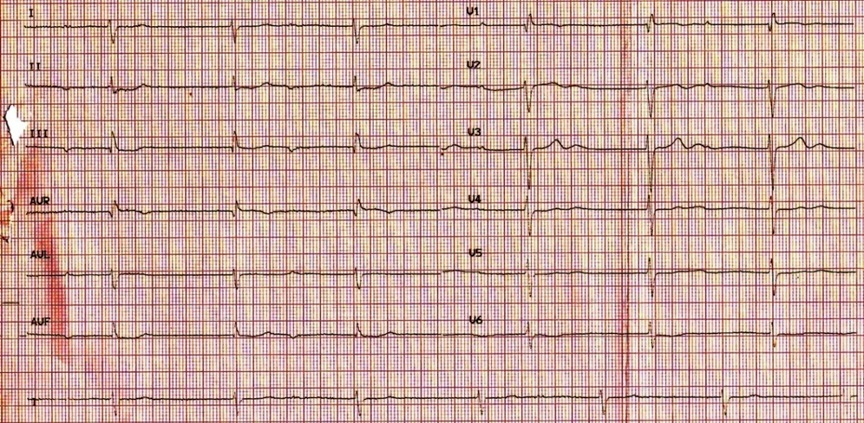
At the admission, her blood pressure was 90/60 mmHg, the heart rate was 42 bpm. Electrocardiogram (ECG) upon admission revealed complete Atrioventricular Block (AVB) and a relatively wide QRS escape rhythm with QRS duration of 115 ms (Figure 1). Echocardiography revealed severe systolic ventricular dysfunction, Right Ventricular (RV) dilation with systematic RV ejection fraction of only 33% assessed by single plane Simpson rule. Significant tricuspid valve regurgitation was also observed. Indexes of interventricular and intraventricular dyssynchrony were not studied.
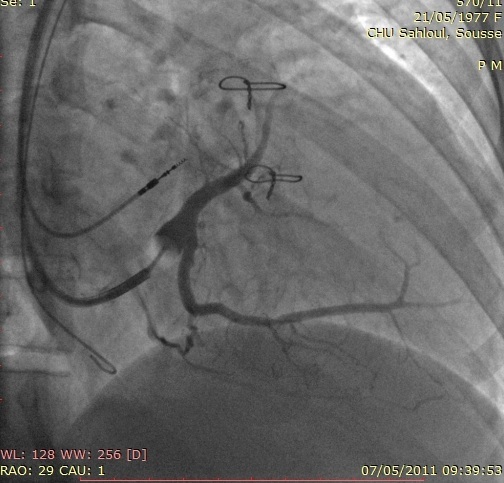
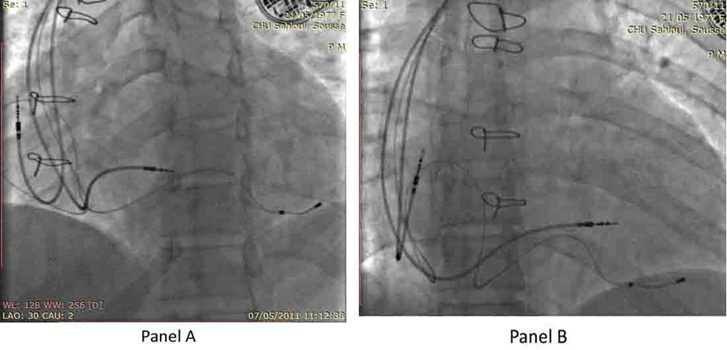
Given the underlying ccTGA with a systemic RV and the complete AVB requiring conventional pacemaker therapy, which in the presence of ventricular dysfunction and conduction disease may further compromise cardiac performance, a biventricular pacemaker (CRT-P) was implanted. After cannulation of the Coronary Sinus (CS), an occluded CS venogram was obtained (Figure 2) with a balloon tip catheter. A specially designed over the wire CS pacing lead (Attain OTW 4193, Medtronic, USA) was then advanced over the wire into the Posterolateral Cardiac Vein (PLCV). A Medtronic 5076 screw-in lead was placed in pulmonic Left Ventricle (LV) septum. Subsequently, a bipolar active fixation lead (Capsure 5076, Medtronic) was positioned at the right atrium appendage. No phrenic nerve stimulation at maximal output was noted. The pacing and measurement of bioelectrical parameters at all the leads appeared satisfactory. These three leads were attached to a Medtronic pacemaker Syncra CRT C2TR01 (Figure 3 panel A and B).
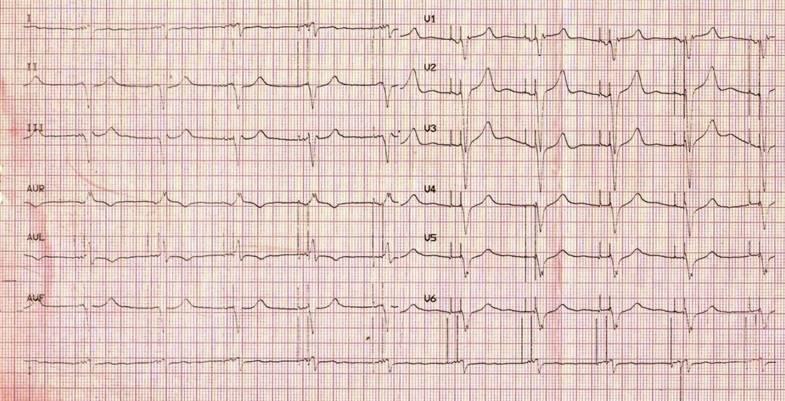
Immediately after the introduction of CRT pacing, the sinus rhythm was reestablished and atrio-ventricular synchronization was achieved. The postoperative ECG showed an atrial and ventricular paced rhythm with a negative paced QRS configuration in DI lead and QRS duration of 130ms (Figure 4). The Atrio-Ventricular (AV) delays were programmed as follows: AV delay sensed at 110ms, AV delay paced at 130ms, without VV delay.
Subsequently, the patient was discharged from hospital and put on captopril, carvedilol, spironolactone and aspirin. The follow up was carried out every six months following the procedure. Postoperatively, the patient achieved total relief from her dizzy spells and her exercise tolerance improved from New York Heart Association functional Class III to II with a significant increase of her systemic Right Ventricle Ejection Fraction (RVEF = 45%) evaluated six months later. During follow-up, both atrial and ventricular lead parameters were all within satisfactory ranges and no arrhythmias was observed.
Within a 48 months follow up, the patient achieved successfully two pregnancies after resynchronization therapy. The mode of delivery was vaginal. In the case of the first pregnancy, she developed active labor at 37 weeks, and had a spontaneous vaginal delivery of a small for gestational age infant with a congenital heart disease. A postnatal diagnosis of an aortopulmonary window was made in the offspring who died 3 days after surgery. For the second pregnancy after resynchronization therapy, both women tolerated labor and childbirth without complication. The second infant had no evidence of structural heart disease on fetal or postnatal echocardiography or physical examination.
The RV ejection fraction was stable after the two pregnancies and estimated to 43%. She remained active and had NYHA class II symptoms on medical regimen.
Discussion
We described a case of cardiac resynchronization therapy in 33 years old women with congenitally corrected transposition of the great arteries with complete AVB and systemic ventricular dysfunction who achieved six successful pregnancies and vaginal deliveries, four before and two after cardiac resynchronization therapy. The main CRT trials on congenital heart disease and paediatric patients have included highly heterogeneous populations [7]. Entirely transvenous CRT system implantation has been described to be feasible in patients with ccTGA [5-7].
The benefit of CRT in the treatment of HF in a systemic RV remains unknown, although limited data suggest trends toward modest improvement in RV ejection fraction and NYHA class. Reverse RV remodeling after CRT has not yet been shown. Data on the efficacy of CRT in a systemic RV consist only of retrospective cohort series; no prospective data are available [8].
The results of cardiac resynchronisation therapy in the systemic right ventricular population are mixed. A total of five studies have reported cardiac resynchronisation therapy results in 68 patients with all studies noting a reduction in QRS duration, but clinical improvement ranged from 25 to 100% over a 4- to 19-month median duration. [5,6,9-11].
The sub-optimal response to cardiac resynchronisation therapy in the systemic right ventricle may be secondary to changes in myocardial fibre arrangement and contraction patterns compared with the left ventricle [12]. In a recent study of carefully pre-selected patients with both inter- and intra-ventricular dyssynchrony by tissue tracking, all patients responded favourably to cardiac resynchronisation therapy; however, this small group accounted for only 10% of the right ventricular systemic population followed-up at the investigators’ institutions [11].
In clinical practice, the indication for CRT in these patients is predominantly electric dyssynchrony because there are no established ways to determine mechanical dyssynchrony in a systemic RV [8].
In our patient, indexes of inter and intraventricular dyssynchrony were not studied. In fact, in this patient with levo-transposition of the great arteries with RV dysfunction, complete atrioventricular block further exposing the myocardium to electrical and mechanical dyssynchrony with lifelong pacing. Right Ventricular Outflow Tract (RVOT) pacing could be enough to preserve a good hemodynamic in this patient but cardiac resynchronization therapy was preferred.
A large number of patients with a systemic RV will require conventional pacemaker therapy which in the presence of ventricular dysfunction and conduction disease may further compromise cardiac performance [13]. Alternative primary pacing strategies such as biventricular pacing may need consideration in this vulnerable group already highly prone to mortality from systemic RV failure [14].
After CRT, the patient showed an increase in here RV EF (from 33% to 45%) without deterioration during the follow up, particularly after the two pregnancies. To the best of our knowledge, this is the first reported case of pregnancies in a women with resynchronization therapy for failure of the systemic right ventricle and complete AV block. Increasing numbers of women with ccTGV are now reaching their childbearing years, and some are becoming pregnant.
Pregnancy induced changes in the cardiovascular system - including a 50% increase in blood volume, increased cardiac output, reduction in cardiovascular resistance, and rapid fluid shifts during delivery- may lead to right ventricular failure in women with a systemic RV which may not adequately respond to the demands placed upon it during pregnancy. A potentially RV dysfunction in this setting can be irreversible [15], and thus pregnancy in women with a systemic RV carries a risk of both short- and long-term maternal and neonatal complications [2-4].
Kowalik E et al., [12], identified 20 pregnancies in 13 women among the 26 women with ccTGA who were followed at their study center during 21 years. There were no pregnancy related maternal deaths. Cardiovascular complications during pregnancy and childbirth occurred in 3 patients (16% of successful pregnancies). Two women developed supraventricular arrhythmias at the end of the second trimester; however, they were observed and required no pharmacologic treatment. One patient required premature delivery at 37 weeks for documented deterioration of right ventricular function. Another patient with complete heart block without pacemaker underwent prophylactic temporary pacing during delivery. Only in one case, congenital heart disease was diagnosed in the offspring. With regard to long-term follow-up, no differences were found in terms of heart failure admissions, pharmacologic treatment, deaths, or echocardiographic parameters compared with non-pregnant women with ccTGA [4].
Gelson et al., [3] reported no cases of congenital heart disease in the offspring but sixteen neonatal complications occurred in 12 (63%) index pregnancies. One stillbirth occurred, five pre-term births occurred (26%) and 10 (53%) babies were small for gestational age.
In our case, an aortopulmonary window was reported in the 5th child of this patient. This congenital heart defect has not been described in the offspring of women with corrected transposition of the great arteries.
Little is known about the incidence of cardiac defects in the offspring of women with ccTGA. Because of the small sample size and a lack of examination in all infants and unsuccessful pregnancies, the maternal transmission of congenital heart disease could not be assessed precisely [4]. Previously, only one case of congenital heart disease had been identified in a live newborn (hypoplastic left heart syndrome) [2].
Conclusion
Adults with congenital heart disease now form the largest group of women with cardiac disease becoming pregnant in the developed world. Cardiac resynchronization therapy demonstrated favorable clinical outcomes in congenitally corrected transposition of the great arteries and can allows pregnancies in women. Each woman with congenital heart disease presents a unique pattern of challenges for the cardiologist, obstetrician, and anesthesiologist, and their care should be tailored to deal with their individual circumstances.
Conflict of Interest
The authors have no conflicts of interest.
Author’s Contribution
SO and SK conceived of this case report. RG and EN helped to draft the manuscript. All authors read and approved the final manuscript.
Figures
Figure 1: Electrocardiogram (ECG) on admission showing complete heart block with a relatively wide QRS complex escape rhythm.
Figure 2: Coronary Sinus (CS) angiogram showing the CS tributaries in Right Anterior Oblique view 30 (RAO 30°).
Figure 3: The location of the atrial and ventricular leads in Left Anterior Oblique view (LAO 30°) (Panel A) and antero-posterior view (Panel B).
Figure 4: ECG after resynchronization therapy.