Introduction
Allergic Rhinitis (AR) is usually referred to as the body's exposure to allergens in the environment, mainly mediated by IgE type. Allergic diseases are a nasal mucosa of chronic non-infectious diseases [1]. The expression is an imbalance of immune response in the body; Th2 cell dominance differentiation and Th1/Th2 cell ratio changes with various inflammatory mediators involved in the allergic disease.
Micro RNA (miRNA, miR), which belongs to a kind of endogenous non-coding RNA and through the 3 noncoding region combined with target genes mRNA, make its mRNA to protein of the translation process, after the transcription of gene regulation in the expression of target genes [2]. IL-4 can promote cell proliferation of related cell humoral immunity and lL-4 can enhance the expression of adhesion molecules of related lymphocytes; as a result, IL-4 can be regarded as the important correlation factor for the advantage differentiation of Th2. IL-13 (Interleukin-13) has become the Th2 cytokine and in recent years, the new focal study and plays an important role in the pathogenesis of allergic rhinitis [3]. TSLP is a kind of IL-7 cytokines, which can stimulate the activation, maturation and migration of Dendritic Cells (DC), and thus activate CD4+T cell precursor to Th2 [4]. In the intestinal mucosal immune study, miR-375 can be induced by IL-13, which can regulate the expression of Th2 epithelial factor TSLP and guide a Th2 amplification circuit [3,4]. Therefore, this study intends to determine the relative regulation of IL-4, IL-13 and TSLP of miR-375 and Th2 dominance in mouse nasal mucosa, thus providing a new basis for gene therapy of allergic rhinitis.
Materials and Methods
Laboratory animals and major reagents
4-8 weeks of SPF BALB/c male100 mice, each mouse weighed averagely 20g, purchased from Changsha Tianjin biotechnology Co., Ltd (Certificate: SCXK (Xiang) 2014-0011), the mice were bred at experimental animal center of Hainan Medical College.
Ovalbumin (OVA, V level, A5503 Sigma company, USA); AL (OH) 3 adjuvant (USA Sigma); derived from polyclonal antibody (Abcam Companies in the United States), miR-375 slow virus control and expression vector (the Hang Seng Han Technology co., LTD.), the total RNA extraction kit, reverse transcription kit and fluorescence quantitative detection kit (DP431 KR106, FP205 day biochemical technology co., LTD.), primers (Shanghai Sangon Biological Engineering co., LTD.), Western Blot kit (C600393 Shanghai Sangon Biological Engineering co., LTD.).
Methods
An allergic rhinitis mouse model: This animal protocol has been reviewed by the experimental animal ethics committee of Hainan medical college.
The mice were randomly divided into
1. Normal saline group
2. Allergic rhinitis group
In the sensitization phase, the OVA of 40ug were dissolved in the physiological saline solution constituting 2mg of Aluminum hydroxide and the final volume was 200ul.
The mice were injected with the intraperitoneal injection, the next day, for two weeks. The stimulation phase starts from day 21 and then USES 5% OVA was sprayed for 10 minutes, then USES 20ul of OVA (40mg/ml) to activate the nasal drip, which is stimulated once a day for 7 days. The saline control group was replaced by an equal amount of physiological saline for intraperitoneal injection, nasal drops and atomization, three times in a row.
Symptom scores: The behavioral changes of the experimental mice were observed and recorded in 30min after the last excitation of the nasal cavity.
• For the scratching nose: 1-4 was mild, above 4 was moderate, and the prolonged was severe
• For sneezes: 1-3 times for mild, 4-10 for moderate, 10 and above for severe
• For the snot: A few snot for mild, middle snot for moderate, heavy snot for severe
The normal group and the allergic rhinitis model group had the nasal drops to stimulate the experiment, and the structure showed that the allergic rhinitis animal model was successfully constructed (Table 1). The normal saline group was 0.83 plus or minus 0.25; allergic rhinitis group was scored, 8.56+ +1.64, with statistical difference between the two groups (P<0.05).
After excitation experiment, select 5 Allergic rhinitis group mice and 5 normal saline mice by random number method, collect the nasal mucosa tissue and lung tissue, lung, such as simple slice in paraformaldehyde fixed after biopsy in the liquid, HE dyeing 200 x were observed under electron microscope nasal mucosa and the organization form of lung tissue. The results showed that the mold was successful.
The intervention of slow virus miR-375 expression and control vector: From the mice group of allergic rhinitis, 36 mice with outstanding performance were selected and divided into three groups by table of random number. Twelve mice were selected from the normal group by table of random. Group them into four groups.
1. miR-375 over expression lentiviral vector group
2. Blank lentiviral vector group
3. Normal saline group
4. Allergic rhinitis group
The allergic rhinitis mouse model of allergic rhinitis was prepared by intravenous injection, with 1.5*107, 1×10 8 PFU/ml of LV-miR375 slow virus injection, 50ul injection, 2 days interval. It lasts three times a week. Blank lentiviral vector group was replaced by an equal amount of Blank lentiviral virus for injection. The saline control group and allergic rhinitis group were replaced by an equal amount of physiological saline for injection.
The mRNA detection of IL-4, IL-13 and TSLP in tissues by real-time fluorescence quantitative PCR: An ultraviolet spectrophotometer, 260nm and 280nm absorbance measurement was used to determine the purity and concentration of the extracted total RNA.
An agarose gel electrophoresis was used to detect its integrity, and then reverse transcription into cDNA. SYBR green real-time fluorescent quantitative Reverse Transcription Polymerase Chain Reaction method (qRT-PCR) was then used to detect physiological saline control group; the allergic rhinitis group; miR-375 slow virus group and slow virus expression vector control group.
In the nasal mucosa of each of the 6 mice, IL-4 and IL-13 appeared on mRNA expression, beta act in noted for the eyes only, relative to gene expression in 2-ΔΔ CT value.
• TSLP upstream primers: 5’aag cca gct tgt ctc ctg aa3’
• Downstream primers: 5’tgg tca ttg agg gct tct ct3’
• IL-13 upstream primers: 5’cag cat ggt atg gag tgt gg3’
• Downstream primers: 5’aca gag gcc atg caa tat cc3’
• IL-4 upstream primers: 5’gca acg aag aac acc aca ga3’
• Downstream primers: 5’tgc agc tcc atg aga aca ct3’
• β-actin upstream primers: 5’tgc tgt ccc tgt atg cct ct3’
• Downstream primers: 5’5’ttg atg tca cgc acg att tc3’
The protein detection of IL-4, IL-13 and TSLP by western imprinting: A total amount of 400-mu l pyrolysis liquid containing PMSF was applied to the nasal mucosa specimens. Tissue homogenate at 4℃ for 30min under x, centrifugal extraction for 5 min 4℃ 10000g for protein.
Determination of protein concentration by BCA method (reference); at-20℃ to save protein proportion of 25mu l5 x loading buffer/100mu l protein; 30ug protein was used for SDS-PAGE gel electrophoresis, and the objective protein was transferred to the PVDF membrane to carry out the immune imprinting. Using GAPDH, DAB color, gel imaging system, image scanning and tanon software, the gray value was analyzed.
Statistical analysis
Experimental data are measured data, with mean +/- standard deviation (+/- s), SPSS 17.0 is used for statistical data analysis, group comparison using single factor analysis of variance. The difference between the two compared with LSD-t test is P<0.05.
Results
Behavioral observations and grading of mice
After the last stimulation, the mice in the allergic rhinitis group showed obvious torsion, sneezing and runt, with the total score of more than 5 points. The control mice of the saline control group had no sneeze, runny nose, and occasionally had a scratching nose. The total scores of both groups were less than 5 points (Table 1, Figure 1 and 2).
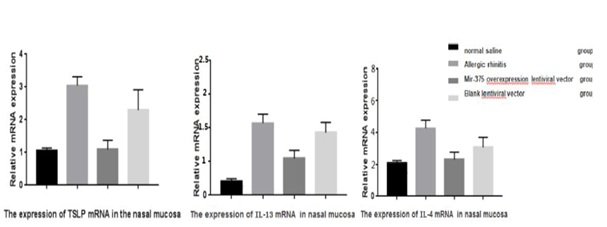
The expression of TSLP, IL-4, IL-13 mRNA in nasal mucosa
The total RNA extracted from nasal mucosa is of high purity and good integrity. miR-375 slow virus of mice express IL-4 in the nasal mucosa, IL-13, arrives at mRNA expression relatively lower than that of allergic rhinitis group and slow virus vector control group, the difference was statistically significant (P<0.05), and no difference with normal saline control group. Allergic rhinitis and slow virus vector controls in the nasal mucosa in mice IL-4, IL-13, arrives at the mRNA expression were had no difference between groups, and relatively were higher than in normal saline control group (Figure 3 and 4). The difference was statistically significant (P<0.05) (Table 1).
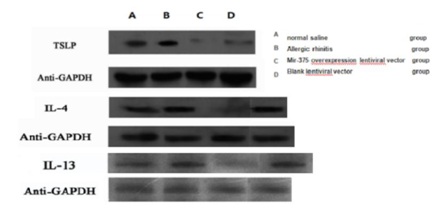
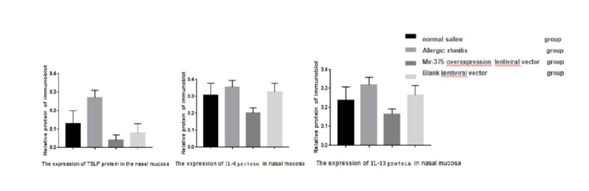
The expression of TLSP, IL-4 and IL-13 protein in nasal mucosa
Compared to saline control group, the group of mice of allergic rhinitis nasal mucosa of IL-4, IL-13 and the virus express group which arrived at protein relative expression in nasal mucosa in mice were lower than allergic rhinitis group and the control group (Figure 5). The difference was significant (Figure 6) (P<0.05).
Discussion
Allergic rhinitis is a common chronic inflammation of upper respiratory tract; in recent years, the prevalence of allergic rhinitis in adult and children showed a trend of rapid increase.
According to the statistics data in Mainland China, population prevalence was 4%~38%, and this may be attributed to the living condition; change in lifestyle, industrialization and other relevant factors such as air pollution [5].
Under normal circumstance, the nasal mucosa is in a state of relatively balanced Th1 and Th2 cells. When the nasal mucous membrane contacts an antigenic substance such as viruses or bacteria, it stimulates the Th1 cells differentiation and secretion of IL-2, IFN-gamma, such as cytokines, and which enhance the cellular immunity resistance to infection.
When susceptible body makes contact, it is atopic allergens. Allergen by Antigen Presenting Cells (APC) recognition, hand over the initial T cells, further differentiation to the Th2 cells, secretion of IL-4, IL-13, such as cytokines.
These cytokines can induce B-lymphocytes into plasma cells; secrete specific IgE, induction of eosinophil’s local accumulation.
Furthermore, specific IgE receptors on the surface of the mast cells and basophils (FC epsilon RⅠ, FC epsilon R) combined with the sensitization, IL-4 and IL-13 in the Th2 cells play an important role.
Again, when the body contacts the allergens, sensitization of mast cells and basophils degranulation caused release of inflammatory mediators, acting on the nasal mucosa sensory nerve, blood vessel walls and glands, causing symptoms such as sneezing, nasal congestion and runny nose [6].
Early link in allergic rhinitis, nasal mucosa epithelial cells through the secretion arrives at the induced DC activation and expression of CD80 and CD86, CD40 and OX40L stimulus molecule, DC strongest APC as antigen presenting function, the expression of OX40L and T cell expression of tumor necrosis factor receptor super family member OX40, initial T cell proliferation and coordinated stimulus to Th2 cell differentiation [7,8]. Therefore, TSLP plays an important role in signaling between epithelial cells, DC and effector lymphocytes: such as asthma [9], topic dermatitis [10] and other allergic diseases.
TSLP over expression can also be detected in nasal mucosa in patients with severe allergic rhinitis, and significantly correlated with IL-4 level [11]. In vitro TSLP-DC stimulated initial CD4+T cell activation and differentiation into Th2 cells, secreting a large amount of IL-4 and IL-13. The co-stimulation signal of OX40L-OX40 is the key signal of the differentiation of initial T-cell to effector T cells [12,13]. The mouse T cells were apoptosis after the OX40 gene knockout, which could not further proliferate and differentiate into effector T cells. When IL-12 exists, OX40L does not induce the formation of Th2 cells. The IL-4 secreted by Th2 cells can strengthen the co-stimulation signal of OX40L-OX40 and promote the production of Th2 cells. However, TSLP’s regulatory mechanism remains unclear by stimulating the expression of OX40L by stimulating DC. Studies have reported that CYT387 block JAK/STAT signaling pathway can cut the allergic rhinitis OX40L expression, reduce arrived-DCs for initiation to Th2 CD4+T cells the ability of cell differentiation, inhibit arrived-type Th2 inflammation induced by DCs [14].
MicroRNA also plays an important role in the differentiation and activation of Th cells. It is reported that the expression of miR-375 has been reduced in Th2 related diseases such as atopic dermatitis [15] and ulcerative colitis [16]. The expression of miR-375 was also reduced in the endobronchial and esophageal epithelial cells after IL-13 stimulation [17]. Biton M et al., found that IL-4, IL-13, can stimulate expression of miR-375 and regulate TSLP expression in HT-29 human colon cancer cells.
However, in esophageal epithelial cells, Lu TX et al., did not find the relationship between miR-375 expression of TSLP and the expression of IL-4, IL-13 and TSLP [11,18]. OVA building AR mice were used, allergic rhinitis and slow virus vector controls were showing obvious symptoms of scratching nose, sneezing and runny nose. Superposition method recorded a total of more than five (5) points, suggested building success. Compared with the expression group of miR-375 slow virus, the symptoms were reduced and the total score was less than 5, indicating that the expression of miR-375 gene had a soothing effect on AR symptoms. The qRT-PCR detection of IL-4, IL-13, arrived at mRNA expression, relatively slow miR-375 viruses express group is lower than the allergic rhinitis and slow virus vector control group, compared with the saline control group, there was no difference. Then over expression of miR-375 in the nasal mucosa in mice to downgrade IL-4, and the expression of IL-13.
The protein expression of Il-4 and Il-13 was detected by Western Blot, and the expression of Mir-375 was lower than that of allergic rhinitis.
Allergic rhinitis group was higher than that of normal saline control group; which further illustrate the above.
Conclusion
The results indicated that the Lentivirus in the nasal mucosa of mice with allergic rhinitis may affect the expression of IL-4, IL-13 and TSLP, but the expression of Lentivirus in the nasal mu-cosa of mice with allergic rhinitis is different from that in the miR-375 Lentivirus group 2. The expression of Lentivirus in the nasal mucosa of mice with allergic rhinitis was different.
The over expression of miR-375 gene in nasal mucosa of allergic rhinitis mice may play a negative role in the expression of IL-4, IL-13 and TSLP, but the relationship between IL-4, IL-13 and TSLP in nasal mucosa of allergic rhinitis mice is still to be more studied. With the development of microRNAs technology, gene targeting technology and molecular biology technology, new diagnosis and treatment related will also improve the present situation of the refractory disease. So the research of the pathogenesis of allergic rhinitis on microRNA can found a new way for diagnosis and treatment of the disease.
Figures
Figure 1: The nasal septum and nasal septal nasal mucous epithelial cells were arranged in order, the cell structure was normal, the cytoplasm was intact and the organelles were clearly visible.
Figure 2: In the nasal and septal eosinophilic granulocytes, the cell lines are irregular and irregular, and mast cells and macrophages are seen.
Figure 3: Common agarose gel electrophoresis of the total RNA of each group (normal saline group, allergic rhinitis group, miR-375 over expression lentiviral vector group, blank lentiviral vector group).
Figure 4: The expression of TSLP mRNA, IL-13and IL-4 in nasal mucosa.
Figure 5: Allergic rhinitis nasal mucosa in mice tissues of TSLP, IL-4 and IL-13 proteins molecularly imprinted images, above corresponding is normal saline group, allergic rhinitis group, miR-375 over expression lentiviral vector group, blank lentiviral vector group’s protein bands and Anti-GAPDH internal protein bands.
Figure 6: The protein expression of TLSP, IL-4 and IL-13 in nasal mucosa.