
A Novel Food Synergy Booster - Research on Its Beneficial Effects by Antioxidant Analysis and Cell Biology
*Corresponding Author(s):
Peter C DartschDartsch Scientific GmbH, Institute Of Cell Biological Test Systems, Wagenfeld, Germany
Tel:+49 54449801322,
Email:pc.dartsch@dartsch-scientific.com
Abstract
Dietary supplements are products which are intended to supplement one’s diet and are not considered to be food. Especially food synergy research has been investigating the entity of foods in their complex natural form and composition for around 20 years.
By use of current in vitro methods we examined the beneficial effects of a novel dietary supplement, named Food Energy Booster , on cultured cells. Moreover, the water- and lipid-soluble antioxidants as well as the total antioxidant capacity of the dietary supplement were examined by photochemiluminescence. The Food Energy Booster combines the greatest possible variety of natural vitamins, minerals and trace elements in order to promote a synergistic effect.
The Food Energy Booster exhibited a remarkable high total antioxidant capacity of more than 38,300 µmol/100 g which was distributed in nearly equal parts for water-soluble and lipid-soluble antioxidants. The basal cell metabolism of connective tissue fibroblasts was increased very rapidly at test concentrations ≥ 2.5 mg/ml. The effect started just a few minutes after application of the Food Energy Booster and reached a maximum in the time range between 20 and 30 minutes. Thereafter, the stimulation slowly decreased, but was still above the control value after 90 and 120 minutes. The activity of superoxide anion radicals which were generated by an oxidative burst of functional neutrophils was decreased in a dose-dependent manner. At a test concentration of 2.5 mg/ml which is equivalent to a single dosage of the Food Energy Booster, the radical activity was decreased by about 35%. The maximum decrease in radical activity by more than 60% was achieved at the highest test concentration of 5 mg/ml, which is equivalent to two single dosages.
According to our test results, the synergistic combination of active ingredients of the Food Energy Booster can be recommended to improve and maintain physical performance and well-being in vivo.
Keywords
Cell metabolism; Food synergy; Oxidative stress; Superoxide anion radicals; Total antioxidant capacity
INTRODUCTION
Dietary supplements are products which are taken orally and contain one or more ingredients such as vitamins, minerals, herbs or amino acids and are intended to supplement one’s diet and are not considered to be food. The use of dietary supplements among adults has increased over the past 30 years not only in the United States, but also in many highly industrialized countries of the world [1-3]. Currently, about half of adults in the United States report to use one or more dietary supplements. The use of supplements is related to an improved health situation and lifestyle choices [4]. Athletes use dietary supplements as part of their regular training or competition program [5]. Many beneficial health effects have also been described for dietary supplements in the case of oxidative stress [6-8].
The latest area of nutritional research is food synergy research, which has its origins in the United States. In contrast to previous approaches of medical research to isolate individual active substances from food and to examine their effects on our body, the food synergy research has been investigating the entity of foods in their complex natural form and composition for around 20 years. It is the goal to examine how the phytochemicals as well as vitamins, minerals and trace elements in the food really work together [9-13]. The novel Food Synergy Booster is based on this knowledge and combines the greatest possible variety of natural vitamins, minerals and trace elements in order to promote a synergistic effect.
In the present study we used current in vitro methods to investigate the beneficial effects of the Food Synergy Booster in terms of antioxidant efficacy and stimulation of basal cell metabolism. Both features complement one another, because an increased cell metabolism also yields an increased generation of endogenous superoxide anion radicals by the respiratory chain in mitochondria.
MATERIALS AND METHODS
Food Synergy Booster
According to the information of the manufacturer and distributor of the Food Synergy Booster (Millivital GmbH, D-55232 Alzey, Germany; homepage: www.millivital.de), the “phytochemicals in this complex mixture of active ingredients are a real power combination”. It contains 27 different fruit juice concentrates, 7 different vegetable juices, 39 different herbal extracts and vitamin C [14,15]. The active ingredients were selected to act in a synergistic way to yield the best possible bioavailability and efficacy. The liquid Food Synergy Booster , batch no. L13003209, with an expiry date of September 30, 2021, was examined in this study.
Recommended daily uptake and test concentrations
The recommended daily uptake for an adult person is 9 ml of pure Food Synergy Booster or mixed in water. In times of increased exertion, the daily dosage can be increased to 9 ml twice a day. When considering this daily uptake together with a (theoretical) complete absorption and distribution of the active ingredients within 3.5 liters of blood fluid, the calculated test concentrations are in the range between 2.5 and 5 mg/ml. Although the bioavailability of the active ingredients might vary, we used test concentrations covering this range for cell biological experiments.
Photochemoluminescence measurement of antioxidant capacity
The method used a PHOTOCHEM® (Analytik Jena, D-07745 Jena, Germany) which combines the very fast photochemical excitation of radical formation with the highly sensitive luminometric detection. The antioxidant capacity of individual substances and complex substance mixtures can be measured and thus their ability to inactivate free oxygen radicals.
Basically, the essential point of the measurement method is in the 1,000 times faster rate of the oxidative reactions compared to normal conditions, which is caused by a photochemical excitation of the reacting molecules. Defined free superoxide anion radicals are generated in the measuring system using a specific dye. Free radicals are detected by their reaction with a chemiluminogenic substance and the measurement of the photons produced. In the presence of antioxidant substances, the intensity of the photochemo luminescence is weakened depending on the concentration. The water-soluble antioxidants were measured and quantified separately from the lipid-soluble antioxidants. The results are given in equivalent concentration units of ascorbic acid for water-soluble substances [16] or vitamin E equivalents (Trolox®) for lipid-soluble substances [17,18].
Basal energy metabolism of connective tissue fibroblasts
The experiments were conducted with connective tissue fibroblasts (cell line L-929, ACC-2; Leibniz Institute DSMZ - German Collection for Microorganisms and Cell Cultures, Braunschweig, Germany) and used in passages 109 to 110. The cells were routinely cultured in RPMI 1640 culture medium with 10% growth mixture and 0.5% gentamycin and incubated in an incubator at 37°C and an atmosphere of 5% CO2 and 95% air at almost 100% humidity.
For the experiments, connective tissue fibroblasts were seeded from 80 to 90% confluent mass cultures at a cell density of 20,000 cells/well in 96-well plates (200 µl culture medium/well) and incubated for 24 h to achieve cell attachment and metabolism. Then, culture medium was removed and a reaction mixture consisting of phosphate-buffered saline with calcium and magnesium, 5 mM glucose, the different test concentrations of the Food Synergy Booster and the tetrazolium dye WST-1 (Roche Diagnostics, Mannheimm Germany). The cleavage of the dye is directly related to the activity of the basal cellular metabolism, which also includes the generation of adenosine triphosphate in mitochondria. The optical density was continuously recorded by an Elisareader (BioTek SLx808 with software Gen 5 Version 3.00) as a differential wavelength measurement ΔOD = 450 minus 690 nm and analyzed with Microsoft Excel for the time interval 0 to 120 minutes. Three parallel experiments were conducted for this study.
Generation of endogenous oxygen radicals by functional neutrophils
Human promyelocytes (cell line HL-60, ACC-3, ECACC 98070106; Leibniz Institute DSMZ - German Collection for Microorganisms and Cell Cultures, Braunschweig, Germany) were routinely grown in RPMI 1640 with 10% growth mixture and 0.5% of gentamycin and incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Subcultures were differentiated to functional neutrophils by the addition of 1.5% dimethylsulfoxide to the culture medium for 6 days. Then, cells are capable of undergoing an oxidative or respiratory burst upon phorbol ester stimulation [19,20].
After 6 days, cells were collected by centrifugation (5 min at 190 x g), washed twice with phosphate-buffered saline by resuspending and centrifugation and were finally resuspended in phosphate-buffered saline with calcium and magnesium containing 10 mM glucose. Cell suspensions were added to the reaction mixture consisting of the different concentrations of the Food Synergy Booster, phosphate-buffered saline with 30 mM glucose, phorbol 12-myristate 13-acetate and WST-1 as tetrazolium dye (all from Sigma-Aldrich, Deisenhofen, Germany). As already described in detail previously, the course of superoxide anion radical inactivation as produced by the stimulated functional neutrophils was monitored by cleavage of the dye [21-24]. A differential measurement of the optical density at 450 minus 690 nm for 30 min at 37°C using a BioTek ELx 808 ELISA reader with software Gen 5 Version 3.00 was undertaken. Three parallel experiments were conducted for this study.
STATISTICAL ANALYSIS
Statistical analysis of the cell biological data was done by using the parameter-free two-sided Wilcoxon-Mann-Whitney test and assumed as significant at p ≤ 0.05.
RESULTS
In accordance with the statements of the manufacturer, the Food Synergy Booster exhibited a remarkable total antioxidant capacity which was about 50% for the water-soluble antioxidants and 50% for the lipid-soluble antioxidants (Table 1). The total antioxidant capacity was more than 38,300 µmol/100 g. For comparison, the following total antioxidant capacity values (each in µmol/100 g) were achieved under the same experimental conditions: ginger - 28,000; marjoram - 9,100; red paprika - 4,200.
|
Water-soluble antioxidants Ascorbic acid equivalents |
Lipid-soluble antioxidants Vitamin E equivalents (Trolox®) |
Total antioxidant capacity |
|
|
34.8 µg/mg = 18,612 µmol/100g |
47.63 µg/mg = 19,759 µmol/100 g |
82.43 µg/mg = 38,371 µmol/100 g |
Table 1: Presentation of the total antioxidant capacity of the Food Synergy Booster. Note that about 50% of the total antioxidant capacity is due to the water-soluble antioxidants and the remaining 50% to the lipid-soluble antioxidants.
The examination of the basal cell metabolism of connective tissue fibroblasts showed that the addition of the Food Synergy Booster to the reaction mixture caused a very rapid and pronounced increase in cellular metabolism at test concentrations ≥ 2.5 mg/ml (Figure 1). The maximum stimulation was a multiple of the control value for the highest test concentration of 5 mg/ml. The effect started just a few minutes after application of the Food Synergy Booster and reached a maximum in the time range between 20 and 30 minutes. Thereafter the stimulation slowly decreased; but was still above the control value after 90 and 120 minutes. The difference to the control value for the two highest test concentrations was highly significant for the time range 10 to 30 min (p ≤ 0.01). For the highest test concentration of 5 mg/ml, all time values up to 120 minutes differed significantly from the control.
As shown in figure 2, the activity of superoxide anion radicals which were generated by an oxidative burst of the cells, was decreased in a dose-dependent manner. Even at the lowest test concentration of the Food Synergy Booster of 0.1 mg/ml, the decreased radical activity was statistically significant (p ≤ 0.05). At the test concentration of 2.5 mg/ml which is equivalent to a single dosage of the Food Synergy Booster, the radical activity was decreased by almost 35% (p ≤ 0.01). The maximum decrease in radical activity by more than 60% was achieved at the highest test concentration of 5 mg/ml which is equivalent to two single dosages per day.
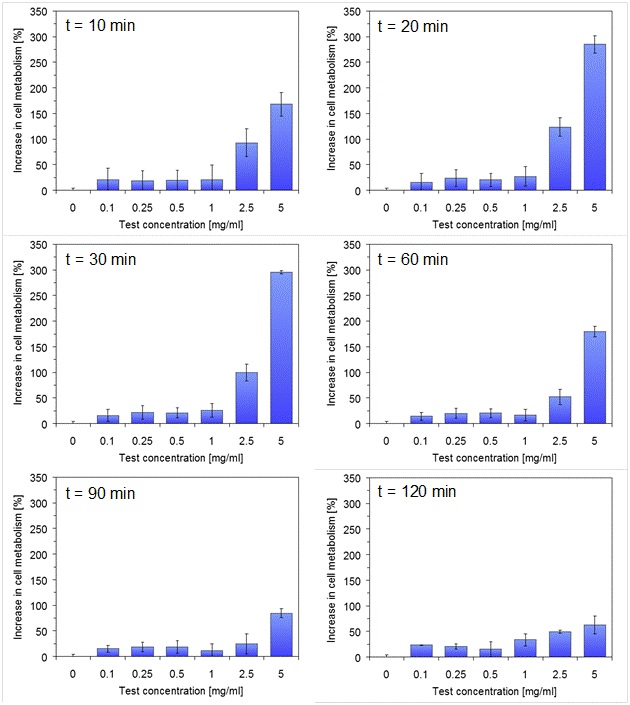 Figure 1: Increase of basal metabolism of cultivated connective tissue cells with time after application of different concentrations of the Food Synergy Booster to the reaction mixture. The untreated control (test concentration = 0 mg/ml) was set as 0%. A single dosage of the Food Synergy Booster corresponds to a test concentration of 2.5 mg/ml with an assumed absorption of 100%. Data represent mean value ± standard deviation of 3 parallel experiments.
Figure 1: Increase of basal metabolism of cultivated connective tissue cells with time after application of different concentrations of the Food Synergy Booster to the reaction mixture. The untreated control (test concentration = 0 mg/ml) was set as 0%. A single dosage of the Food Synergy Booster corresponds to a test concentration of 2.5 mg/ml with an assumed absorption of 100%. Data represent mean value ± standard deviation of 3 parallel experiments.
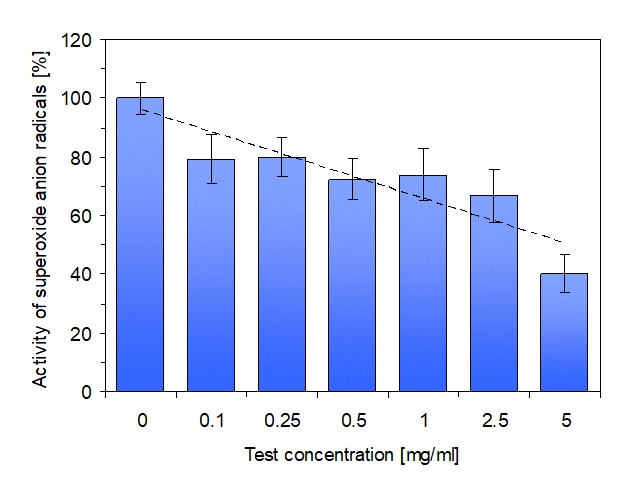 Figure 2: Presentation of the measurement data for the activity of superoxide anion radicals in the reaction mixture. The untreated control (test concentration = 0 mg/ml) was set as 100%. The dose-dependent decrease of superoxide anion radical activity can be clearly seen. A single dosage of the Food Synergy Booster corresponds to a test concentration of 2.5 mg/ml. For a better visualization of the dose-dependent efficacy of the Food Synergy Booster, the calculated linear regression is also depicted. Data represent mean value ± standard deviation of 3 parallel experiments.
Figure 2: Presentation of the measurement data for the activity of superoxide anion radicals in the reaction mixture. The untreated control (test concentration = 0 mg/ml) was set as 100%. The dose-dependent decrease of superoxide anion radical activity can be clearly seen. A single dosage of the Food Synergy Booster corresponds to a test concentration of 2.5 mg/ml. For a better visualization of the dose-dependent efficacy of the Food Synergy Booster, the calculated linear regression is also depicted. Data represent mean value ± standard deviation of 3 parallel experiments.
DISCUSSION
Since many years it is well known that mitochondria play a central role in the energy metabolism of cells, because they transform a part of the energy derived from the oxidation of food into adenosine triphosphate. A dietary supplement as tested here with such a remarkable efficacy on cell metabolism in vitro should also increase physical performance in vivo. This is one reason why it is called Food Synergy Booster. However, an increased cellular metabolism and the increased generation of adenosine triphosphate by the respiratory chain in mitochondria also produces increased amounts of endogenous superoxide anion radicals, which are the precursors to other highly reactive oxygen species such as hydrogen peroxide and hydroxyl radical [25].
Reactive oxygen species are compounds that are naturally produced in the human body. They can possess positive effects (for example on the immune system) or negative effects (for example lipids, proteins or DNA oxidation and damage) [26-29]. To limit these unwanted and harmful effects, an organism has its antioxidant system. This system consists of antioxidant enzymes such as superoxide dismutase, catalase, glutathione peroxidase and non-enzymatic antioxidants such as various vitamins [30]. An imbalance between endogenous radical generation and antioxidant defence leads to oxidative stress, which may be involved in aging and in a number of pathological processes [31-33].
The inactivation of endogenously generated superoxide anion radicals is the second goal of the Food Synergy Booster . On one hand it is able to increase cell metabolism very efficiently and on the other hand it contains ingredients in form of phytochemical and vitamin C which possess a synergistic effect and are able to inactivate the resulting excess of reactive oxygen radicals circulating in the blood [34,35]. However, it cannot be answered from our test results whether the generation of superoxide anion radicals by the cells was inhibited or the increased inactivation of radicals which were released into the reaction mixture. Regardless of this consideration, the superoxide anion radical concentration was reduced effectively. The effect is also demonstrated by the high total antioxidant capacity as examined here for the water-and lipid-soluble antioxidants of the dietary supplement. Besides this point, phagocytic cells such as polymorphonuclear neutrophils, are able migrate from the blood into the tissue and produce a local overload of reactive oxygen radicals in the course of inflammatory processes [31,36].
In conclusion, the Food Synergy Booster tested in this study has clearly demonstrated its potential to increase basal energy metabolism of connective tissue fibroblasts immediately after application. Moreover, its total antioxidative capacity and its potential for the inactivation of endogenously generated reactive oxygen species is very prominent and helps to reduce oxidative stress. According to our test results, the synergistic combination of active ingredients of the Food Synergy Booster can be recommended to improve and maintain physical performance and well-being in vivo.
REFERENCES
- Foote JA, Murphy SP, Wilkens LR, Hankin JH, Henderson BE, Kolonel LN (2003) Factors associated with dietary supplement use among healthy adults of five ethnicities: the Multiethnic Cohort Study. Am J Epidemiol 157: 888-897.
- Harrison RA, Holt D, Pattison DJ, Elton PJ (2004) Are those in need taking dietary supplements? a survey of 21 923 adults. Br J Nutr 91: 617-623.
- Rock CL (2007) Multivitamin-multimineral supplements: who uses them? Am J Clin Nutr 85: 277-279.
- Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT (2013) Why US adults use dietary supplements. JAMA Intern Med 173: 355-361.
- Maughan RJ, Depiesse F, Geyer H (2007) The use of dietary supplements by athletes. J Sports Sci 25: 103-113.
- Shahidi F (2012) Nutraceuticals, functional foods and dietary supplements in health and disease. J Food Drug Anal 20: 226-230.
- Bouayed J, Bohn T (2010) Exogenous antioxidants - Double-edged swords in cellular redox state. Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev 3: 228-237.
- Pham-Huy LA, He H, Pham-Huy C (2008) Free radicals, antioxidants in disease and health. Int J Biomed Sci 4: 89-96.
- Jacobs DR, Murtaugh MA (2000) It’s more than an apple a day: an appropriately processed plant-centered dietary pattern may be good for your health. Am J Clin Nutr 72: 899-900.
- Messina M, Lampe JW, Birt DF, Appel LJ, Pivonka E, et al (2001) Reductionism and the narrowing nutrition perspective: time for reevaluation and emphasis on food synergy. J Am Diet Assoc 101: 1416-1419.
- Jacobs DR, Steffen LM (2003) Nutrients, foods, and dietary patterns as exposures in research: a framework for food synergy. Am J Clin Nutr 78: 508-513.
- Jacobs DR Jr, Tapsell LC (2007) Food, not nutrients, is the fundamental unit in nutrition. Nutr Rev 65: 439-450.
- Jacobs DR Jr, Gross MD, Tapsell LC (2009) Food synergy: an operational concept for understanding nutrition. Am J Clin Nutr 89: 1543-1548.
- King CG (1936) Vitamin C, ascorbic acid. Physiol Rev 16: 238-262.
- Nowak D, Go?li?ski M, Wojtowicz E, Przygo?ski K (2018) Antioxidant properties and phenolic compounds of vitamin C-rich juices. J Food Sci 83: 2237-2246.
- Shosuke K (2004) Vitamin C: basic metabolism and its function as an index of oxidative stress. Curr Med Chem 11: 1041-1064.
- van den Berg R, Haenen GRMM, van den Berg H, Bast A (1999) Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem 66: 511-517.
- Zulueta A, Esteve MJ, Frígola A (2009) ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem 114: 310-316.
- Teufelhofer O, Weiss RM, Parzefall W, Schulte-Hermann R, Micksche M, et al. (2003) Promyelocytic HL60 cells express NADPH oxidase and are excellent targets in a rapid spectrophotometric microplate assay for extracellular superoxide. Toxicol Sci 76: 376-383.
- Peskin AV, Winterbourn CC (2000). A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1). Clin Chim Acta 293: 157-166.
- Dartsch PC (2006) TIIOS – a sensitive and cell-based test assay for the screening of biologically active substances for their antioxidant potential. Innov Food Technol 32: 72-75.
- Ishiyama M, Shiga M, Sasamoto K, Mizoguchi M, He P-G (1993) A new sulfonated tetrazolium salt that produces a highly water-soluble formazan dye. Chem Pharm Bull 41: 1118-1122.
- Ishiyama, M, Tominaga H, Shiga M, Sasamoto K, Ohkura Y, et al. (1995) Novel cell proliferation and cytotoxicity assays using a tetrazolium salt that produces a water-soluble formazan dye. In Vitro Toxicol 8: 187-190.
- Tan AS, Berridge MV (2000) Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt WST-1 to produce a soluble formazan: a simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J Immunol Methods 238: 59-68.
- Lenaz G, Bovina C, Formiggini G, Parenti Castelli G (1999) Mitochondria, oxidative stress, and antioxidant defences. Acta Biochim Pol 46: 1-21.
- Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82: 47-95.
- Frisard M, Ravussin E (2006) Energy metabolism and oxidative stress. Endocrine 29: 27-32.
- Finaud J, Lac G, Filaire E (2006) Oxidative Stress : Relationship With Exercise and Training. Sports Med 36: 327-358.
- Bergamini CM, Gambetti S, Dondi A, Cervellati C (2004) Oxygen, reactive oxygen species and tissue damage. Curr Pharm Des 10: 1611-1626.
- Laursen PB (2001) Free radicals and antioxidant vitamins: optimizing the health of the athlete. Strength Cond J 23: 17-25.
- Kuhn MA (2003) Oxygen free radicals and antioxidants. Am J Nurs 103: 58-62.
- Halliwell B (1994) Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet 344: 721-724.
- Ward PA, Warren JS, Johnson KJ (1988) Oxygen radicals, inflammation, and tissue injury. Free Radic Biol Med 5: 403-408.
- Bendich A, Machlin LJ, Scandurra O, Burton GW, Wayner DDM (1986) The antioxidant role of vitamin C. Adv Free Radical Biol Med 2: 419-444.
- Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee et al. (2003) Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr 22: 18-35.
- Nathan C (2002) Points of control in inflammation. Nature 420: 846-852.
Citation: Dartsch PC, Obenland K (2020) A Novel Food Energy Booster - Research on Its Beneficial Effects by Antioxidant Analysis and Cell Biology. J Med Stud Res 3: 017.
Copyright: © 2020 Peter C Dartsch, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

