
Breastfeeding: History, Techniques, Benefits, Complications and Care
*Corresponding Author(s):
Gabriel Barreda-GómezResearch And Development Division, IMG Pharma Biotech, 48160 Derio, Bizkaia, Spain
Tel:+34 944316577,
Fax:+34 946013455
Email:gabriel.barreda@imgpharma.com
#Equal Contribution
Abstract
Human Breast Milk (HM) is the natural food produced by the mother to nourish the newborn and is considered the best food to feed infants to ensure their optimal growth and development. Milk, precisely, is a food with unique characteristics that allows the mother to transmit her defense mechanisms to the newborn, while the act of breastfeeding strengthens the mother-child relationship. Furthermore, there is large evidence that HM is composed by different bioactive agents that can modulate brain development, the immune system and the function of the gastrointestinal tract. Therefore, the World Health Organization, as well as the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN), recommends to feed infants exclusively with human breast milk at least during their first 6 months of live to achieve optimal growth, development and health, and later, up to two years of age or older, along with complementary foods.
However, in some situations, breastfeeding is partially or totally impossible, for that reason, Infant Formulas (IFs) may be used to feed infants. The aim of these IFs is to mimic human’s milk composition, even if it is almost impossible due to its complexity, to provide the babies with their specific nutritional needs to ensure their proper growth.
The purpose of this article is to review the history of breastfeeding, breastfeeding techniques, its benefits and complications and also to compare human breast milk composition to infant formula’s composition.
The History of Breastfeeding
The history of breastfeeding begins from the point where it has been the only way to ensure the health and survival of the newborn, and its failure, one of the fundamental causes of infant mortality. Breastfeeding is a physiological activity that is part of the female reproductive process that defines mammals. However, unlike in other mammals, breastfeeding is a social construct in humans. Therefore, it depends on learning, beliefs, norms, social class, ethnicity, place where they lived, etc., among other socio-cultural conditioning factors that evolve and involute over time [1].
Although it is a topic that is present in different treatises by philosophers, doctors and historians, the female view of it is almost completely lacking. References about breastfeeding are frequent, but indirect and with little information on practices. Thus, the most studied topic is that of wet-nurses. In ancient civilizations there were political codes that made it compulsory to give breast milk to children. However, in the second half of the 20th century, this practice has diminished to the point of disappearing in several areas of the world. Effect caused because of the introduction of synthetic milk [1].
As is well known, in every age and culture breastfeeding has been determined by various factors. These include social relations, cultural needs, myths, role, feminine aesthetics, socio-economic status, etc. The main variant that existed in breastfeeding was that of giving mother's milk through wet nurses. Between the 10th and 14th centuries in Europe a stable activity of breastfeeding is known. In the following centuries, from the 15th to the 19th century, mercenary breastfeeding was a flourishing practice. In some countries, legislation in favor of breastfeeding was even developed [1].
The prehistory of breastfeeding
It goes without saying that breastfeeding has been around since the beginning of mankind, and even before. Humans are homeothermic, "warm-blooded" vertebrate mammals with hair and milk-producing mammary glands. The ability to regulate body temperature was an essential feature that allowed mammals to survive in cold places and to develop nocturnal activity [1].
To emphasize that providing their offspring with a nutritious food that benefits their growth and development provided them with improved survival rates. Even beyond its nutritional characteristics, breastfeeding allowed them to protect the lives of their children at the time of their greatest vulnerability and to ensure the learning of vital functions; in addition to the primary bond of attachment, which evolved into the one we know today [1].
Breastfeeding in antiquity
In ancient civilizations such as Mesopotamia and Babylon, there were political codes that required breastfeeding. In ancient Babylonia, breastfeeding was recommended until an advanced age, around three years old. In the Code of Hammurabi (1800 B.C.), there are rules about wet nurses, regulating the pay and ways of offering this service [1].
It was considered an honorable job, and also regulated the sexual customs, hygiene and social life of these women. In Egypt, breastfeeding was also extended to the first three years of life. Breast milk was the irreplaceable food, and guaranteed a home life for infants under the care and love of their mothers [1].
Breastfeeding in Egypt ratified and consolidated affectionate bonds between mother and child, with a lasting character, embodied in the literary and funerary tradition. The appointment of the wet-nurse who ruled in Pharaonic Egypt helped to elevate and support the social rank of women, with the wet-nurse of the future pharaoh being the highest echelon. Literary and iconographic depictions of scenes of the suckling of the king by various divinities can be found, and it is even possible to find in the Isis "Lactans" the model for the representation of the Virgin Mary with her Divine Son in medieval art [1].
Among the Greeks, breastfeeding was also considered a primordial practice. In Sparta, only those sons of the king who had been fed by their mother could attain the throne. Plutarch (1st century BC) relates the story of the possession of the throne by the second son of King Themistes, only because the first-born son had been fed with milk from a stranger. Hippocrates (400 BC), in Aphorisms, classifies the ages of life into seven, naming the first as infancy or the age of lactation [1].
Breastfeeding in Greece
The Ebers Papyrus contains detailed descriptions of the care of newborns, breastfeeding and even breastfeeding positions. It also describes lactational diseases, infant mortality due to parasites and criteria for determining the quality of milk. However, wet nurses were very common in classical Greece [1].
Aristotle (384-322 BC) in his History Animalium is interested in lactation and describes methods for determining whether a woman's milk, whether from her own mother or from a wet nurse, is good for the infant, coming to the conclusion that the milk of the first days, or colostrum, should not be drunk by the newborn. Most noblewomen in the Roman Empire relied on wet nurses to nurse their children [1].
Soranus of Ephesus (2nd century AD), considered the father of gynecology for his treatises on women's diseases, explains in detail the conditions for choosing a good wet nurse, her diet, lifestyle and ways of breastfeeding. Describing childcare practices, he defines breastfeeding and teething as the most important events in the life of a child [1]
Wet nurses were highly sought after by the upper class, as not only could they gain a better social status by becoming a wet nurse, but also the family that hired them acquired a certain prestige in the community. The wet nurses were no longer considered slaves because of the bond between them and their children, who grew up healthier and stronger [1].
Breastfeeding in Europe
From the 13th to the 19th century, in France, upper-middle class women did not breastfeed their children, but gave them wet-nurses, animal milks and cereal preparations. In Italy, specifically in Florence, around year 1300, the custom spread of sending children of the urban noble and middle classes with a wet nurse to the countryside for about 2 years [1].
In Rome, although the practice of breastfeeding was encouraged, it was associated with premature ageing, wear and tear and dilatation of the breasts. During the Renaissance, it became widespread in Europe, especially in Italy and France, that most lower-class women breastfed more than one child at a time: their own child and the child in their care [1].
In Spain, especially in big cities, breastfeeding was a salaried activity, which generated a market for wet-nurses, in which various forms of activity coexisted: women who raised children in their own homes in rural areas, close to provincial capitals; women who lived in big cities and also raised children at home, while doing other work at home; salaried wet nurses in private homes, who formed part of their domestic service and who, in turn, paid other women to look after their child [1].
However, in the first decades of the 20th century, as we know, wet nurses had a long tradition in Spain, but their role was increasingly being questioned except in exceptional cases. Doctors, pediatricians, educators, intellectuals and all those sensitized by the child protectionist trend considered that, under normal conditions, the best thing for newborns was for them to be breastfed by their own mothers and not by other women, who charged fees for doing so [1].
Breastfeeding in the 21st century
Nowadays, if mother and baby are healthy, regardless of the type of birth they have had, it is important that the newborn is placed on top of its mother, in close skin-to-skin contact and allow them both to maintain that contact, without interruption or interference, at least until the baby has had its first breastfeed, and ideally for as long as mother and baby wish [1].
Newborn babies have innate abilities that, if we let them, they will start to develop at birth. When placed face down on its mother's abdomen, thanks to its senses (especially touch and smell) and its reflexes, it is able to reach its mother's breast on its own. It will crawl up to it, smell it, touch it with its hands and then with its mouth and finally, it will be able to latch on to the breast spontaneously, with its mouth wide open, covering the nipple and a large part of the areola [1].
Skin-to-skin contact is not only important for the successful establishment of breastfeeding, but also helps the newborn to adapt better to life outside the womb and to establish an emotional bond with its mother. Therefore, early skin-to-skin contact should be encouraged in all newborns regardless of the type of feeding they will receive later [1].
From a nursing point of view, hospital routines such as identification and Apgar test can be performed while the baby is on top of the mother. Weighing, vitamin K administration, eye prophylaxis and hepatitis B vaccination can wait until after the first two hours of skin-to-skin contact or after the first breastfeeding has been completed. These procedures are uncomfortable and painful for the baby, but if they are done while the baby is suckling, the pain and discomfort of the pricks can be reduced [1].
Breastfeeding Techniques
While mother and child are in hospital, nurses instruct mothers in breastfeeding techniques, such as latch-on techniques and breastfeeding posture.
Good latch-on
A good breastfeeding technique prevents complications such as cracks and pain, empties the breast correctly and allows an adequate milk supply for each baby [2,3].
For a good latch-on, it is important that the baby's whole body is facing the mother, and that when she opens her mouth she inserts a large part of the areola, especially at the bottom (where her chin is) so that when she actively moves her tongue, she does not injure the nipple. Signs of a good latch are: the baby's chin touching the breast, the mouth is wide open, the lower lip is turned outwards (everted) and the cheeks are rounded or flattened (not sunken) when suckling. In addition, more areola should be visible above the mouth than below. If the baby is well latched on, breastfeeding does not hurt. Pain is a sign that, in most cases, indicates an incorrect latch or posture [2,3].
Breastfeeding positions
There is no single suitable position for breastfeeding, what is important is that the mother is comfortable, that the latch is suitable, and that the baby is facing and attached to the mother's body [2].
Biological breastfeeding position
The biological nurturing position is based on the study of maternal and neonatal reflexes. The mother will be placed lying down (between 15° and 65°) on her back and the baby on his stomach, in close skin-to-skin contact with the mother's body. This position allows the baby to free himself from his weight and to develop crawling and searching reflexes. It also ensures that the baby's face is in contact with the breast. The mother helps the baby to reach the breast by offering limits with her arms. This position triggers a series of reflexes in both of them that will facilitate a good latch, efficient feeding and better milk production [2].
 The biological breastfeeding position can be used at any time, but it is particularly suitable during the first few days and when there is a latch-on problem (pain, cracks, rejection of the breast, etc.) [2].
The biological breastfeeding position can be used at any time, but it is particularly suitable during the first few days and when there is a latch-on problem (pain, cracks, rejection of the breast, etc.) [2].
Sitting breastfeeding position
The sitting position is usually more comfortable with the feet elevated on a stool, also a little reclining, in the biological parenting position, in case of episiotomy, as leaving that area out of the chair greatly relieves discomfort [2].
The baby should be placed with the trunk facing the mother. In this way the mother can support the baby with one hand on his back, resting his head on her forearm. With the other hand, she can direct the breast towards the baby's mouth and when the baby opens his mouth, gently bring him closer to the breast so that he can grasp a good piece of the areola. This is the most commonly used position after the first few days, when the mother is more mobile and confident with the baby. It is not necessary to try to hold baby's bottom with the hand of the arm on which the baby is resting, as this usually forces the baby's head too close to the elbow, which can cause the neck to flex or not be able to stretch backwards, making it more difficult for them to latch on and swallow comfortably [2].

Lying down position
In this position, the mother lies down on her side, with her head slightly elevated (on a pillow) with the baby also on his side, lying down on the bed, with his body facing her and close to her body. The mother brings the baby to her breast by gently pushing him on his back when he opens his mouth to facilitate the latch-on [2].
 This is a very comfortable position for night feeds and the first few days, although it is usually more uncomfortable and less effective than the biological feeding position [2].
This is a very comfortable position for night feeds and the first few days, although it is usually more uncomfortable and less effective than the biological feeding position [2].
Inverted or rugby ball position
In this position, the baby is placed under the mother's armpit with the legs backwards and the head at breast level, with the nipple at nose level. It is a very comfortable position to breastfeed twins and premature babies. It is important to support the baby's neck and shoulders but not the head, which needs to have its neck stretched back (deflected), allowing it to latch on to the breast better and swallow more comfortably [2].
Piggyback position
With the mother in a sitting position, the baby sits on one of the mother's legs, with the abdomen close to the mother’s and supported by her. This position can be helpful in cases of cleft lip or cleft palate, small jaw (retromicrognathia). This position can help in cases of cracks and in babies with severe gastro-esophageal reflux, prematurity, cleft lip or cleft palate, small jaw (retromicrognathia) or hypotonia. In these cases, it may be necessary to support the breast from underneath while supporting the baby's chin [2].

Breast Care Tips for Breastfeeding
Breasts and nipples care throughout breastfeeding is essential to keep mother’s health. One of the parts of the body that changes the most during pregnancy and, subsequently, during breastfeeding is the breast. From the first trimester it undergoes substantial changes (for example, the size of the bust or the color of the nipples changes) and it is important to take specific care of it.For this reason, some of the tips for breast care are specified below:
- Wash breasts only with water when bathing or showering. The small bumps (Montgomery glands) in the areola produce an oil that moisturizes and protects the nipples. Soaps and shower gels can wash away this natural oil, causing dryness and irritation.
- Let nipples air dry or rub them gently with a towel.
- It is not necessary to clean breasts and nipples before breastfeeding. In fact, the bacteria on the surface of the breast can help baby's gut microbiome to develop [4].
- Fresh breast milk can help heal damaged nipples, so massage in a few drops of milk before and after feedings.
- Change breast pads frequently if they become wet to reduce the risk of yeast or bacterial infections, such as thrush.
- Don't increase the time between feedings to give your nipples a "rest". Your baby needs to feed on demand to stay healthy and grow properly. Remember that frequent feedings allow you to generate and maintain your milk supply, so keep feeding despite the pain.
Useful nipple care products
- Nipple cream made with ultra-pure lanolin: a natural product obtained from sheep's wool. It moisturizes and promotes healing of the nipples. It is safe for your baby, so you do not need to remove the lanolin before breastfeeding.
- Hydrogel patches can be placed on sore nipples to instantly relieve pain and create ideal conditions for healing. You can even store them in the fridge to keep them cool.
- Nipple shields are placed inside the bra. They are perfect for preventing clothes from rubbing against sore nipples and have holes to let air into the nipples to help them heal.
- Nursing bras, made of a breathable material, such as cotton, or a fabric that dries quickly and wicks excess moisture away from damaged nipples.
Nipple shields are silicone covers that fit over the nipples and have small holes that allow breast milk to flow through when breastfeed. They protect the skin and can give babies who are not latching on properly something firmer to latch on to. In general, liners should be considered a short-term solution. If problems arise or you experience pain, see your lactation consultant or lactation consultant.
Breastfeeding Complications
Despite better care breastfeeding, sometimes can have some "setbacks" that do not depend on the mother's good will but on external factors, such as: ankyloglossia, frenulum labialis, pain .... and most seriously mastitis.
Ankyloglossia
Ankyloglossia is a disorder suffered by 4-11% of newborns. It occurs when the strip of skin that attaches the tongue to the bottom of the mouth (lingual frenulum) is too short. A baby with ankyloglossia will not be able to open his mouth wide enough to grasp all the breast needed during feeding, and his tongue will probably not cover his lower gum when sucking. This can lead to sore nipples for you and frustration for your baby. This condition can be treated with a simple procedure called a frenotomy. This intervention, performed by a healthcare professional, usually does not require anesthesia and can help solve breastfeeding problems immediately [5,6].
Labial frenulum
There is also a similar, though less common, condition called frenulum labialis, in which the frenulum that joins the upper lip to the upper gum is too short. These two disorders are not always detected in neonatal checks so be vigilant and seek the advice of a health professional for detection [7].
Pain
Breastfeeding is always pleasant, however, the first few days you may feel pain or discomfort when the baby latches on to the breast. The most frequent cause of nipple pain is the baby's poor positioning, sucking only the nipple, not the whole breast. This pain is transitory and usually disappears within a week [5].
Cracks
The cracks are usually caused by the baby's poor posture with respect to the breast, compressing the mother's nipple with his gums. In addition to the pain, bleeding or bloody vomit may appear. Cracks only require cleaning the nipple after each feeding and correcting the position so that the baby's mouth covers a large part of the areola and not just the nipple. Soft silicone liners may be helpful in some cases [8].
Breast engorgement
Breast engorgement or engorgement is normal from the third day of labor onwards, because in those days milk production increases rapidly and the baby may be unable to express all of it. Engorgement is uncomfortable but decreases or disappears by the end of the first week after birth. Sometimes the tension of the breasts is so hard that the baby cannot latch on, in which case you should press with the fingers of both hands on the areola, at the base of the nipple, trying to sink your hand for a few minutes. In this way, a few drops of milk will appear, which will stimulate the baby's sucking and at the same time allow him to better grasp the nipple [8].
Flat nipples
Some women have small nipples, so flattened, that the baby may have difficulty catching, latching on and holding the nipple in his mouth. You can help by squeezing the breast areola hard with your thumb and forefinger. This allows the nipple to come out of the breast, making it easier for the baby to latch on to the nipple [5,8].
Not having enough milk
This is surely the most frequent cause of breastfeeding failure, the mistaken belief that the mother does not have enough milk or that the composition of the milk is not adequate, is watery or incomplete. It is known that the composition of breast milk is maintained even in case of maternal malnutrition because it is the insurance for the maintenance of the species [9].
In summary, more and more advantages of breastfeeding are being discovered every day, both for the baby and the mother, and these benefits are not only evident during the months of breastfeeding, but persist throughout childhood and beyond. Despite this, premature withdrawal from breastfeeding is all too common and is usually justified on the wrong or false grounds [5,9].
Mastitis
Mastitis is the inflammation of part of the breast. It is generally caused by the accumulation of milk produced by the obstruction of a duct that transports the milk to the nipple, or by a failure to empty all the milk produced; it can later become infected. In both cases, with or without infection, there is pain that will improve at the end of the feeding and increase at the start of the next one. There may also be redness and increased heat in the painful area, sometimes fever. In no case does mastitis justify the withdrawal of breastfeeding, nor when it occurs with high fever or when the mother receives antibiotics to cure it [5,8].
Benefits of Breastfeeding
Breast milk is an ideal, complete and healthy food for newborns and children under 2 years or more; for containing more than 300 nutrients and exact amounts of fats, sugars, water, proteins and vitamins that the child needs to grow and develop, which in turn provides many benefits for the baby, the mother, the family, society and to companies and institutions in the public and private sectors, such as [10-12]:
Benefits for baby
- It has antibodies that protect from prevalent childhood diseases such as: diarrhea, allergies, asthma and respiratory infections.
- Decreases the risk of prevalent childhood diseases such as: diarrhea, asthma, pneumonia, allergies, among others.
- Reduces the risk of malnutrition.
- Reduces the risk of sudden death by 1.5 to 5 times.
- Contains the necessary nutrients for optimal growth.
- It is easy to digest, which reduces colic in babies.
- It has sufficient fluids and electrolytes for hydration.
- It has the best bioavailability of iron, calcium, magnesium and zinc.
- Promotes emotional and intellectual development and prevents future mental health problems.
- Helps to develop clear language early.
- It has long-term effects on health by reducing the likelihood of developing chronic diseases such as obesity, diabetes, cardiovascular disease, type 1 and type 2 diabetes, leukemia and hypercholesterolemia in adulthood.
- It causes babies to achieve better brain development that will allow them to perform better in school which will provide them with better economic opportunities in life.
- It creates an emotional bond with the mother through which breastfed children grow up happier, more secure and more emotionally stable.
- Protects against tooth decay and reduces the risk of orthodontic treatment in childhood and adolescence.
Benefits for the mother
- Creates an affectionate mother-baby bond, which favors the development of self-esteem, healthy personality and high levels of intelligence at later ages.
- Aids rapid recovery after childbirth.
- Helps to burn extra calories, which allows for rapid recovery of pre-pregnancy pressures.
- Prevents post-partum depression.
- In the long term it prevents osteoporosis as well as breast and ovarian cancer.
- It reduces the risk of postpartum bleeding and therefore reduces the risk of developing anemia.
- In the mother's body it produces special hormones that help her feel relaxed and loving towards her baby.
Benefits for the family
- It feeds the baby at any time as it is always available and within the reach of any economy.
- It does not have to be bought, nor does it need to be prepared or stored.
- It favors family savings by not having to spend on milk formulas, bottles, dummies and other utensils to prepare it.
- Reduces health care costs for the baby, as the child is less likely to get sick.
- Saves time in formula preparation, washing and sterilizing bottles.
Benefits to society
- It is a way to invest in society's human capital, as breastfed children perform better in school and, have better opportunities for professional development.
- It avoids the consumption of paper, plastic, aluminum or petrol used in preparing, wrapping or transporting formula milk.
Benefits for public or private companies/institutions
- Substantially improves the health of the mother and her child, reducing the occurrence of illness in the first year by up to 35%.
- It reduces the turnover or loss of qualified personnel due to the birth of a baby, which saves on recruitment and training of new personnel, as well as the time needed for optimal performance.
- Reduces the cost of health care.
- Reduces the number of medical leaves of absence for the worker or her child, as well as sick leave to care for her or her child.
- Improved overall emotional state of the worker during the workday and in her personal life.
- Increased loyalty and sense of belonging of the workers by providing them with facilities to continue to feed their child.
- Facilitates return to work at the end of maternity leave and reduces the need for extra leave to care for the baby.
- It facilitates the combination of maternal responsibility with long-term employment.
- It positions the company as a socially responsible employer.
Improves the employer's public image because it takes care of the welfare of women workers and their families, and makes the company more attractive to potential employees and investors.
Human Breast Milk Composition
Human’s milk composition has been extensively investigated in terms of macro and micronutrients [13]. In figure 1 can be seen that HM is a complex fluid composed by water, carbohydrates, proteins, fat, vitamins, minerals, digestive enzymes and hormones [14,15].
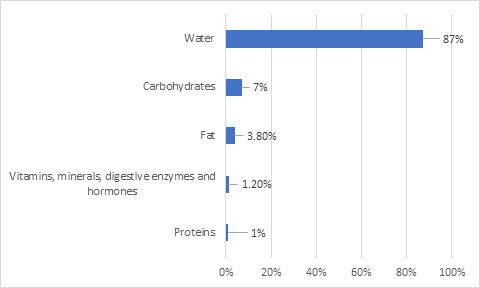 Figure 1: HM macro and micronutrient composition distribution.
Figure 1: HM macro and micronutrient composition distribution.
The World Health Organization, as well as the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN), recommends to feed infants exclusively with human breast milk at least during their first 6 months of live to achieve optimal growth, development and health [16,17] and later, up to two years of age or older, along with complementary foods. HM besides being the perfect nourishment for infants, it also represents a link between the mother and the infant due to the supply of different bioactive components in HM such as lactoferrin, immunoglobulins, cytokines, hormones, Human Milk Oligosaccharides (HMOs), antioxidants and growth factors [13].
HM’s another function is to promote the adaptation of infants to the environment that surrounds them [13]. Furthermore, HM also helps in establishing circadian rhythm in infants. This is due to the variations in nutritional and bioactive components that HM suffers during the day [18].
There are different stages of lactation. The first fluid produced by mothers after infant’s birth is named as colostrum and it’s produced during the first few days. Colostrum is produced in very low quantities, and it’s appearance is more yellowish than mature HM. This is because it is rich in immunologic components (secretory IgA, lactoferrin, leukocytes) and it has low concentrations of lactose. This is a biological advantage to infants, as first the gain immunological function and then nutritional [19]. Few days after infant’s birth (usually
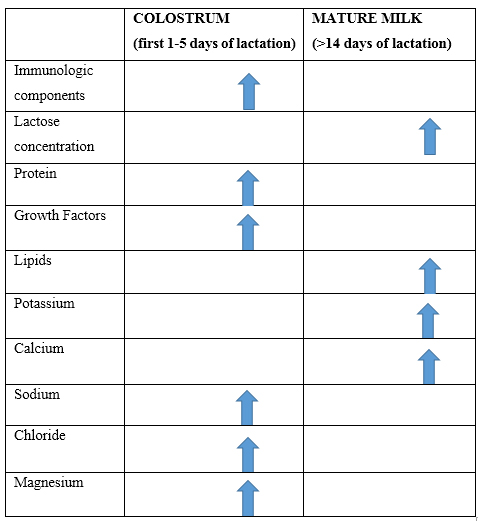 Table 1: Comparison between colostrum and mature milk composition.
Table 1: Comparison between colostrum and mature milk composition.
There is a high intra- and inter-variability in woman regarding the composition of HM [21,22]. The composition of HM is influenced by many factors, the most significant are maternal status (maternal diet and nutritional status) and the stage of lactation [13,23,24]. Mother’s diet can modulate the composition of HM, being micronutrient (vitamins and minerals) and fat content the most variable depending on mother’s diet [25,26] and in a lesser way there can be variations in lactose and proteins [25]. For example, HM EPA and DHA concentrations can change depending on maternal dietary EPA and DHA intake [27,28]. In some studies, have been seen that the mothers in lactation stage living near sea (where it’s supposed to be a higher intake of fish) have higher EPA and DHA values in HM [14,27]. Whereas n-6 fatty acids as ARA aren’t modulated by diet [29]. What is more, fat concentrations tend to be higher while breastfeeding has longer duration and it also varies during the day. This is a biological benefit to infants as first they get a HM rich in essential nutrients and in those who are hungrier/thirstier get a HM rich in fat to satisfy their caloric needs [25].
Human milk’s lipids
Lipids in HM are diverse and extremely complex although it is known that the major components of HM fat are triacylglycerols (TAGs 98%), phospholipids (PLs 0.8%) and cholesterol (0.5%) [27,28,30]. Despite the fat content in HM is not the highest (~3,8%), it’s the major source of energy, providing 50% of total Energy to the infant [14,25,27]. In addition, human’s milk fat supplies the infant with essential Fatty Acids (FAs) and liposoluble vitamins [31,32]. Around half of fatty acids present in HM are saturated fatty acids, being palmitic acid (16:0) the most abundant saturated fatty acid (23% of total fatty acids) [28,32]. Most of the palmitic acid present in HM is located in the central position (sn-2 position) of TAGs which makes them easier to be digested and absorbed [25,27,31]. In contrast, in plant oils usually used in infant formulas as fat source, palmitic acid is uncommonly located in sn-2 position as it is usually located in the outer carbons of the glycerol [32]. Second most abundant FAs are monounsaturated fatty acids (MUFA), where oleic acid (36%) (18:1w9) and palmitoleic acid (16:1 w7) are dominant [27,28]. This two MUFAs are usually located in SN-1,3 positions [30] and they are in higher concentrations in HM than in cow’s milk [33].
Beside these types of fats, HM also contains essential fatty acids, polyunsaturated fatty acids (PUFAs) and long chain PUFAs (LC-PUFAs), as linoleic acid (C18:2w6) (15%) and in less proportion alpha-linolenic acid (C18:3w3) (0.35%)(28). Arachidonic acid (ARA, C20:4w6) and eicosapentaenoic acid (EPA, C20:5w3) are respectively synthesized from these two fatty acids in the fetus and after birth, although is limited because of the premature enzyme activity of the child [28]. Thereby, the required amounts of ARA and DHA of the infant must come directly from the mother through placenta during pregnancy (in the last three months of pregnancy), or through HM after birth [28]. Additionally, EPA is further converted into docosahexaenoic acid (DHA, 22:6w3) [28]. These last three fatty acids (EPA, ARA and DHA) play an essential role in different processes in neonates as the correct growth, inflammatory responses, immune function, cognitive development and motor system [28].
Breckenridge et al., found that HM does not contain FA with less than 10 carbons. This can be seen also in dog and Guinea pig milk [33].
At present, lipidomics is widely used to make a comprehensive analysis of the lipidome of human milk. Due to these techniques, many studies have researched TAG composition in HM around the world. Pons et al. found 24 different TAGs in Spanish HM, whereas Tu et al., Zhao et al., and Linderborg et al., found more than 60 TAGs in Chinese and Finish HM, respectively [23,34-36]. In a recent study (2019), Zhang et al found more than 90 different TAGs in Chinese human milk; being Oleic-palmitic-linoleic the most abundant TAG in human milk [37].
Regarding PLs, have lately gained interest due to their positive impact on cognitive development of infants [30]. For example, Lindahl et al., found 31 different PLs in colostrum and mature human milk [38] and Liu et al., found 70 different PLs species [39]. The main classes of PLs present in HM (exactly in Milk Fat Globule Membrane (MFGM)) are Phosphatidylethanolamine (PE), Phosphatidylcholine (PC) and Sphingomyelin (SM). There are also other PLs present in lower amounts in HM: Phosphatidylserine (PS) and Phosphatidylinositol (PI) [14,40]. SM and PC are also a major source of choline, which is essential for development of the central nervous system [41]. PLs, besides being an important source of energy for the infant, they also provide the infant with LC-PUFAs [40]. MFGM is a complex and unique structure composed mainly of lipids and proteins that surrounds the lipid droplets in milk and it is secreted by the milk-producing cells of humans and other mammals [42]. The main role of MFGM is to provide infants with FAs. MFGM in addition to PLs, is composed by cholesterol (150 mg/L of HM), enzymes, glycolipids and glycoproteins [40].
Lipid content in HM (synthesis, storage and transport) may change by the circadian system [43]. Total milk fat shows a 24-hour rhythm, with the higher concentrations at night compared to other times of the day [18]. What is more, in a recent study, was found that PEs and TAG concentrations are higher in the evening comparing to morning’s concentrations. However, TAGs and DAGs appeared to be the most variable fats in HM [15].
Human milk’s proteins
HM proteins provide the infant with essential amino acids, bioactive proteins and peptides that help to the correct development of neonates [44]. Recent proteomics studies, found that colostrum contains higher concentrations of lactoferrin, BSSL, and lysozyme than mature HM [45,46].
Protein concentrations vary in HM depending on lactation stage. Approximately, crude protein content in colostrum (first 1-5 days of lactation) is 2.5g/100 mL, whereas it decreases in transitional milk (6-14 days of lactation) 1.7 g/100 mL and in mature human milk (>14 days of lactation), 1.3 g/100 mL [47].
There are about 400 different proteins in HM classified in 3 major categories: caseins (suspended in solution), whey proteins (present in solution) and mucins (present in the milk fat globule membrane) [31,44].
Whey proteins
The most abundant whey proteins are α-lactalbumin, lactoferrin, IgS, serum albumin and lysozyme [28,31]. Lactoferrin is a significant protein in HM that constitutes between 15% to 20% of the total protein content. A major part of iron content in HM is bound to Lactoferrin but it is only saturated about 6-9%(45). Lactoferrin’s first bioactivity described in HM was the bacteriostatic effect on E. coli [48]. Lactoferrin has a constant high affinity for iron. Due to this, lactoferrin is able to keep iron out from pathogens that require it, such as E. coli [45]. Lactoferrin also affects to cell proliferation and differentiation and to cytokine expression, by modulating the immune response [45]. In a comparative study made in Italy, was found that high concentrations of lactoferrin stimulate cell proliferation, while low concentrations stimulate cell differentiation [49]. This suggests that lactoferrin is a key modulator of intestinal epithelial development, although further studies are needed.
α-lactalbumin constitutes about 15% of total HM protein. In the mammary gland, α-lactalbumin plays an essential role in the production of HM. Along with a galactosyltransferase enzyme, it forms lactose synthase that is the responsible of lactose synthesis, which drives milk volume [45,50]. Further, α-lactalbumin forms peptides with biological activities during the digestion which makes α-lactalbumin a major source of bioactive peptides and essential amino acids such as tryptophan or lysine [50]. This protein has 2 specific binding sites: one of them for calcium and the other one for essential elements, such as iron and zinc [51]. Thereby, Keller et al., observed in Rhesus monkeys that, α-lactalbumin enriched formulas increased zinc absorption comparing to breastfed animals [52]. In addition, Sändstrom et al., showed a higher absorption of iron in infants fed with α-lactalbumin enriched formulas [53].
Lysozyme enzyme activity is much higher in HM than in other mammalian’s milk. For example, comparing to cow’s milk, HM lysozyme activity is 3000 times higher. The principal action of lysozyme is to kill gram-positive bacteria. For it, proteoglycan’s matrix of the bacterial cell wall is degraded [45].
Caseins
Due to casein, there are three different types present in HM: α-, β- and κ-casein, being β- and κ-casein the most abundant caseins in HM casein micelle [31]. Human breast milk’s amino acid profile changes depending on the stage of lactation. Whey proteins content vary between 80% to 50% of total protein in human breast milk during lactation time, whereas in cow’s milk is around 18%. This translates into a casein rich infant formula, making them harder to digest comparing with human breast milk [28]. Has been proved that casein peptides inhibit some enzymes associated to the inflammatory process [54].
κ-casein, or more precisely its immediate proteolytic fragment, Glycomacropeptide (GMP), can bind to pathogens avoiding infections. Strömqvist et al., found that κ-casein in HM can inhibit attachment of Helicobacter pylori to human gastric mucosa. This translates into a lower incidence of infection by Helicobacter pylori in infants fed by HM [51].
During digestion, β-casein forms different phosphopeptides (CPPs) which some of them bind divalent cations (for example calcium). For this reason, it has been suggested that it may facilitate the absorption of these nutrients. This has only been proved in animal model experiments as there is a lot of controversy in human studies [51]. For example, Sun et al., shown strong calcium absorption capacity of CPPs in rats. No instant, it is still unknown the specific mechanism which promotes calcium absorption [55].
Mucins
Mucins are proteins present mainly in MFGM [44]. In the bovine MFGM, there have been identified a total of 120 proteins, being most of them mucins [56]. On the other hand, Liao et al., identified 191 different proteins in human MFGM in a lactation period of 12 months [57]. Regarding MFGM proteins’ activities, they are usually implicated in beneficial bioactivities [57]. For example, Peterson et al., found that HMGM proteins have antimicrobial bioactivity and Imam et al and also Snow et al. found they had anticancer effects [58-60].
Human milk’s carbohydrates
HM carbohydrates are able to regulate infant’s appetite, breastfeeding patterns, and body composition [61]. There is a wide variety of carbohydrates present in HM, being the most abundant lactose, as it provides the neonate with 40% of total energy intake [31,61]. In fact, lactose is present in higher concentration in humans than in other species, due to the high energy demands of the human brain [31]. Nevertheless, other carbohydrate fractions are also important for an early health of the infant such as monosaccharides (glucose and galactose) as well as Human Milk Oligosaccharides (HMO) [26,61]. HMOs are the third most abundant solid components after lactose and lipids [62]. Although HMOs are indigestible to the infant, are essential for nourishing the gastrointestinal microbiota [31,63]. In addition, HMOs promote gastrointestinal tract’s barrier function and also can change the regulation of the immune system by modulating the expression of the intestinal epithelial genes involved [64]. These bioactive components seem to reduce the adhesion of pathogens’ in the mucosal surface of the host as they are offered as a target to pathogens’ [65]. HMO composition and content can vary between mothers, between ethnicities and during lactation time [61,66].
Human milk’s growth factors and hormones
Growth Factors’ (GF) main function is to backup infants’ growth by the proliferation and differentiation of their immature cells. The main GF found in HM are: Vascular Endothelial Growth Factor (VEGF), Hepatocyte Growth Factor (HGF), Glucagon-Like Peptide-1 (GLP-1), Epithelial Growth Factor (EGF) and Insulin Growth Factors (IGFs) [13,67].
The colostrum, the first milk produced when breastfeeding starts, contains the highest concentrations of GF, to meet the newborns’ needs [13].
The role of several hormones in HM modulating appetite, energy balance and fat mass deposition has been investigated in different studies. The hormones that influence mostly infants’ development (including risk of obesity in adulthood) are: leptin, ghrelin, Insulin Growth Factor 1 (IGF-1), adiponectin and insulin [13].
There are several studies that have investigated the correlation between leptin’s HM concentration and the risk of obesity in adulthood, although discordant results were found. Fields et al., Alderete et al., and Chan et al., found in three different studies, correlations between high leptin and insulin concentrations in HM and a lower Body Mass Index (BMI) in infants [68-70]. These correlations could be temporary as they haven’t been found in infants older than 1 year old [70]. Contrarily, Uysal et al., Galante et al., Logan et al., and Khodabakhshi et al., haven’t found any correlations between leptin concentrations in HM and BMI of infants [71-74].
Ghrelin and adiponectin stimulate hunger on the hypothalamus although Chan et al., and Galante et al., found no association between adiponectin and infants’ body composition [70,72].
Regarding the functions of IGF-1, it is well known that plays an essential role in cell proliferation and apoptosis inhibition. However, further studies are needed to research correlations between IGF-1 present in HM and infants’ weight gain as the results shown in current studies are still discordant [72,75,76]. Galante et al., found that higher HM IGF-1 was associated with higher BMI at 13 months but lower BMI at 3- and 5-years old infants [72]. Whereas Kon et al., found that higher HM IGF-1 was associated with lower BMI at infants 3-months old [76].
Micronutrients
Some micronutrients can vary their content in HM depending on mother’s diet [25]. In some studies, have shown the average content of some micronutrients. For example, potassium and sodium concentrations in HM are around 500 mg/L (80 mg/100 kcal) and 140-160 mg/L (22-25 mg/100 kcal), respectively [77]. Regarding magnesium content in HM, the range found varies from 15 to 64 mg/L (2.3-9.8 mg/100 kcal [78].
On the other hand, calcium concentrations found in HM varied from 200 to 300mg/L (31-46/100 kcal), with a calcium: phosphorus ratio of 2:1 [79,80].
Infant Formula
Infant Formulas (IF) are necessary to feed infants that cannot be breastfeed [28,40], because raw cow’s milk doesn’t contain enough vitamin E, iron or essential fatty acids to provide infant’s optimal growth.
IFs in market are mostly available in three different forms: powder (must be mixed with water before feeding), liquid (must be mixed with an equal amount of water) and ready-to-feed (no mixing is required) [28].
There are many brands of IFs available on the market and each manufacturer has their unique formulation for their product [81]. All these IFs available in the market, are considered completely safe for the health of the infants [82,83]. What is more, manufacturers are in a continuous improving of their products to more closely resemble to HM composition, the gold standard.
IF’s composition is strictly regulated by government agencies, as World Health Organization (WHO), Codex Alimentarius commission, European Food Safety Authority (EFSA) or European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPHGAN) [70], establishing guidelines for all manufacturers that must be precisely followed. This includes requirements for quality and manufacturing practices in their own countries. The most abundant components added to formula, as proteins, lipids or carbohydrates have an established range of minimum and maximum values for their effectiveness and a history of safe use. Moreover, this range must be respected during the life of use of the product [28].
Nowadays, there are many IF options to help infants to meet their nutritional demands even if they have special conditions, metabolic disorders or not [84]. Most of the infant formulas are based on cow’s milk, which is considered the best alternative to HM to feed infants [81,82,84]. Bovine milk contains higher values of fat, minerals and proteins than HM that is why this kind of milk must be skimmed and diluted to be as similar as possible to HM [28]. What is more, cow’s milk based formulas are the most recommended by pediatricians. Besides cow’s milk based formulas, there are also soymilk based formulas and specialized formulas (used to meet special needs) [28,81]. Furthermore, soymilk-based formulas should be given to infants under a professional advice, and aren’t recommended to infants under 6 months as the protein quality is lower than de cow’s milk-based formulas [82]. What is more, around 10% of infants with cow’s milk proteins allergy develop an allergy to soybean proteins too [82]. In 2014, Owens et al. found no evidence between the superiority of one type of IF to another [85].
Energy in Infant Formulas
IF’s should contain between 60 kcal (250 kJ) and 70 kcal (295 kJ) of energy per 100mL [86]. Exactly, cow’s milk–based infant formulas apport around 64 or 67 calories per 100mL [81]. Comparing to HM, exits commonalities in energy densities between cow’s milk based formulas and HM, as HM contains around 70 kcal per 100ml [87].
Lipid content in Infant Formulas
The content of fat in IFs has to be between 4.4 g/100 kcal and 6.0 g/100 kcal. To simulate lipidic profile of HM, a mixing of vegetable oils that have been considered safety (from a toxicological point of view) for infants is added to IF. This includes coco oil, soya oil, palmitic oil etc. Partially hydrogenated vegetable oils should not be used to produce Ifs due to their Trans Fatty Acid (TFA) content [86].
Unlike HM’s lipidic profile, IF lipidic profile is static and doesn’t change in time. HM contains DHA and ARA, LC-PUFAs that play an essential role in different processes in infants as the correct growth. Nevertheless, bovine milk has no content of DHA and ARA [84]. For this reason, LC-PUFAs (mostly DHA and ARA) found in IF are obtained adding fish oil, DHA rich algae oil and ARA rich mushroom oil [88]. The range of DHA recommended to be added to IFs goes from 20mg/100 kcal to 50mg/100kcal [86]. It’s not recommended DHA supplementation without ARA supplementation, as both influence infants’ optimal neurodevelopment. This is because in many studies it has been seen that, when DHA/ARA ratio is 1/≥1, the typical proportion in HM, it has a positive effect on vision maturation and infant’s growth/development [82]. Despite this, it hasn’t been established a correct DHA/ARA ratio by official agencies [86]. If these two LC-PUFAs aren’t added to the IFs simultaneously, it has been seen that it could have a negative impact on infants’ neurodevelopment [89]. It is known that ARA concentration in HM is low. Despite this, compositional requirements of ARA for infant formulas, have not been yet established by government agencies such as ESPGHAN or EFSA [86,90]. These agencies, in accordance to HM’s composition, also suggest that EPA content in IFs should not be higher than DHA content [86].
IF usually contains a significantly lower concentration of cholesterol (0 -50 mg/L) than HM [42]. Moreover, the presence of phytosterols in IF can reduce the absorption of cholesterol through bile salt/lecithin micelle competition [42].
Due to MFGM, many studies have shown the benefits of adding MFGM to IFs, such as reduction of acute otitis incidence [84,91]. What is more, MFGM also plays an important role in infants’ neurodevelopment [84].
Regarding fatty acids, palmitic acid content in IF derived from cow’s milk is higher than in HM [33]. In addition to differences in content of this fatty acid, the positioning palmitic acid in TAGs is also different to the positioning in HM. Unlike in HM, in IF palmitic acid is uncommonly positioned on the sn-2 position which makes them easier to be absorbed. When palmitic acid is positioned on the outer carbons of TAGs, the formation of indigestible soaps is increased which makes them harder to be absorbed [84].
Protein content in Infant Formulas
Protein content in IFs can be added by different sources but not all are permitted. Allowed sources of protein in IF are cow’s milk protein, goat’s milk protein, Isolated Soy Protein (ISP) and protein hydrolysates of unspecified origin and unspecified degree of hydrolysis [86]. Regarding protein content established in IFs, is between 2 and 2.5 g/100 mL and protein/energy ratio <3 g/100 kcal, whereas in preterm infant formulas protein content and protein/energy ratio is higher, 2.9 g/100 mL and 3.5 g/100 kcal, respectively [92]. Usually, IF contain higher values for proteins than HM, as HM contains about 0.90–1.2 g/100 ml [84,93]. Moreover, there are also differences in the protein quality. IF compared to HM contains higher values of casein that makes more difficult to the infant to digest [28]. What is more, bovine caseins are rich in bioactive molecules as HM, although not all infants can metabolize these proteins [84]. Besides the differences in the protein content, thermal processing of IF can also change peptide patterns during digestion [94]. In recent studies, has been proved that infants fed with a high protein content IFs is associated with excess weight gain in infancy, which increases risk of obesity [95,96].
Due to the concentration of whey proteins in IF, is lower than in HM. The major whey protein present in bovine milk is β- lactoglobulin. The high content of this type of whey protein in cow’s milk based formulas could have a negative impact on infants, due to the strong association between β-lactoglobulin and the occurrence of cow’s milk allergy [84]. When protein content of IFs was reduced, α-lactalbumin was added to infant formulas as a good source of tryptophan (essential amino acid in high concentrations in α-lactalbumin) [51]. α-Lactalbumin also helps to the correct development of infants [50]. The only amino acid forms allowed to be added are L because D forms of amino acids can cause D-lactic acidosis [28].
Carbohydrate content in Infant Formulas
The carbohydrates that are allowed to be added in IFs are: lactose, maltose, sucrose, glucose, maltodextrins, glucose syrup, pre-cooked starch and gelatinized starch free from gluten. The minimum and maximum contents of carbohydrates to be added in IFs are established between 9 g/100 kcal (2.2 g/100 kJ) and 14 g/100 kcal (3.3 g/100 kJ) [86].
Regarding lactose, the most abundant carbohydrate in HM, have been established to be added in a minimum content of 4.5 g/100 kcal [86].
HMOs are not present in a natural form in cow’s milk, this is why IFs are supplemented with different prebiotic oligosaccharides: galacto-oligosaccharides, fructo-oligosaccharide, polydextrose, and mixtures of these [82,97]. Adding prebiotic to IFs Gastrointestinal (GI) microbiota is altered in the infant resembling that of breastfed infants [97]. IFs supplemented with these compounds have different beneficials to the infant’s health such as a lower stool pH, a better stool consistency and frequency and higher concentration of bifidobacteria in their intestine [97].
In 1995 Gibson et al., proposed that prebiotics modulate the colonic microbiota and since then they are added to cow’s milk–based IF [98]. Nowadays, 7 oligasacharides are approved to be added in IFs, such as Galactooligosaccharides (GOS), Polydextrose (PDX), Lactulose (LOS), inulin, and Fructooligosaccharides (FOS) [99]. Bettler et al., found that IFs enriched with GOS increased infants’ defecation and stool pH was similar to those fed wit HM [100]. Another study made by Grüber et al., in 2010 showed that adding a ratio of 9:1 of GOS and FOS to IFs, reduced significantly the appearing of early atopic dermatitis in infants [101].
Sucrose, glucose and fructose are simple carbohydrate that should be avoided to add in IFs due to intolerance to fructose and saccharidase deficiency of the infant (28)(86). No instant, sucrose and glucose are allowed to be added to hydrolyzed (partially or totally) IF for gustatory reason [86].
Micronutrients
From a nutritional point of view, the minimum contents of micronutrients established to be added in IFs cover the nutritional needs of healthy infants. Minimum and maximum calcium contents established to be added in IF is between 50 mg/100 kcal and 140 mg/100 kcal, respectively, with a calcium: phosphorus ratio of 1:2. Regarding to phosphorus the range established is between 25 mg/100 kcal and 90 mg/100 kcal, respectively. On the other hand, minimum and maximum magnesium contents in IF range from 5 mg/100 kcal to 15 mg/100 kcal [86].
Sodium and potassium ranges established are 20 mg/100 kcal-60 mg/100 kcal and 60 mg/100 kcal and 160 mg/100 kcal, respectively [86].
References
- TECH Education (2022) Historia de la lactanciamaterna - Blog TECH España Universidad Tecnológica. TECH Education, USA.
- AEP (2022)Recommendations On Breastfeeding Of The Lactation Committee Of The Spanish Association Of Paediatrics. AEP, Spain.
- Cadwell K (2007) Latching-on and suckling of the healthy term neonate: breastfeeding assessment. J Midwifery Womens Health 52:638-642.
- Pannaraj PS, Li F, Cerini C, Bender JM, Yang S, et al. (2017) Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr 171:647-654.
- Berens P, Eglash A, Malloy M, Steube AM, Brodribb W, et al. (2016) ABM Clinical Protocol #26: Persistent Pain with Breastfeeding. Breastfeed Med 11:46-53.
- Segal LM, Stephenson R, Dawes M, Feldman P (2017) Prevalence, diagnosis, and treatment of ankyloglossia: Methodologic review. Canadian Family Physician 53: 1027.
- Shea JEO, Foster JP, Donnell CPFO, Breathnach D, Jacobs SE, et al. (201) Frenotomy for tongue-tie in newborn infants. Cochrane Database Syst Rev 3: CD011065.
- Jacobs A, Dakn MA, Becker K, Both D, Gatermann S, et al. (2013) S3-Guidelines for the Treatment of Inflammatory Breast Disease during the Lactation Period: AWMF Guidelines, Registry No. 015/071 (short version) AWMF Leitlinien-Register Nr. 015/071 (Kurzfassung). Geburtshilfe Frauenheilkd 73: 1202.
- Kent JC, Prime DK, Garbin CP (2012) Principles for maintaining or increasing breast milk production. J Obstet Gynecol Neonatal Nurs 41:114-121.
- Chatterji P, Markowitz S, Gunn JB (2022) Early Maternal Employment and Family Wellbeing. National Bureau of Economic Research, USA.
- Colten R, Mrtek MB, Mrtek RG (1995) Comparison of maternal absenteeism and infant illness rates among breast-feeding and formula-feeding women in two corporations. Am J Health Promot 10:148-153.
- Heymann J, Raub A, Earle A (2013) Breastfeeding policy: a globally comparative analysis. Bull World Health Organ 91: 398-406.
- Vizzari G, Morniroli D, Ceroni F, Verduci E, Consales A, et al. (2021) Human Milk, More Than Simple Nourishment. Children (Basel) 8: 863.
- George AD, Gay MCL, Trengove RD, Geddes DT (2021) Human Milk Lipidomics: Current Techniques and Methodologies. Nutrients 10: 1169.
- Selvalatchmanan J, Rukmini AV,Ji S, Triebl A, Gao L, et al. (2021) Variability of Lipids in Human Milk. Metabolites 11:1-18.
- WHO (2022) Child health and development. WHO, Geneva, Switzerland.
- Agostoni C, Braegger C, Decsi T, Kolacek S, Koletzko B, et al. (2009) Breast-feeding: A commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr 49:112-125.
- Italianer MF, Naninck EFG, Roelants JA, Horst GTJ, Reiss IKM, et al. (2020) Circadian Variation in Human Milk Composition, a Systematic Review. Nutrients 12: 1-16.
- Ballard O, Morrow AL (2013) Human milk composition: nutrients and bioactive factors. PediatrClin North Am 60:49-74.
- Rivers LAN, Dolan LM, Huang B (2012) Timing of stage II lactogenesis is predicted by antenatal metabolic health in a cohort of primiparas. Breastfeed Med 7: 43-49.
- Garwolinska D, Belka WH, Namiesnik J, Wasik AK (2021) Rapid Characterization of the Human Breast Milk Lipidome Using a Solid-Phase Microextraction and Liquid Chromatography-Mass Spectrometry-Based Approach. J Proteome Res 16: 3200-3208.
- Lukacka MC, Olejnik BK, Pawilowicz MO (2018) Breast Milk Macronutrient Components in Prolonged Lactation. Nutrients 10: 1893.
- Tu A, Ma Q, Bai H, Du Z (2017) A comparative study of triacylglycerol composition in Chinese human milk within different lactation stages and imported infant formula by SFC coupled with Q-TOF-MS. Food Chem 221: 555-567.
- Jura AB, Senczyna AC, Oledzka G, Wegierek DS, Weker H, et al. (2018) Maternal Nutrition and Body Composition During Breastfeeding: Association with Human Milk Composition. Nutrients 10: 1379.
- Koletzko B (2016) Human Milk Lipids. Ann NutrMetab 69: 28-40.
- Berger PK, Plows JF, Demerath EW, Fields DA (2020) Carbohydrate composition in breast milk and its effect on infant health. Curr Opin Clin NutrMetab Care 23:277-281.
- Delplanque B, Gibson R, Koletzko B, Lapillonne A, Strandvik B (2015) Lipid Quality in Infant Nutrition: Current Knowledge and Future Opportunities. J Pediatr Gastroenterol Nutr 61: 8-17.
- Martin CR, Ling PR, Blackburn GL (2016) Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients 8: 279.
- Salem N, Dael P (2021)Arachidonic Acid in Human Milk. Nutrients 12: 626.
- Zhang X, Liu L, Wang L, Pan Y, Hao X, et al. (2021) Comparative Lipidomics Analysis of Human Milk and Infant Formulas Using UHPLC-Q-TOF-MS. J Agric Food Chem 69:1146-1155.
- Andreas NJ, Kampmann B, MehringDoare KL (2015) Human breast milk: A review on its composition and bioactivity. Early Hum Dev 91: 629-935.
- Demmelmair H, Koletzko B (2018) Lipids in human milk. Best Pract Res Clin Endocrinol Metab 32: 57-68.
- Breckenridge WC, Kuksis A (1967) Molecular weight distributions of milk fat triglycerides from seven species. Journal of Lipid Research 8: 473-478.
- Pons SM, Bargalló AC, Folgoso CC, LópezSabater MC (2000) Triacylglycerol composition in colostrum, transitional and mature human milk. Eur J ClinNutr 54: 878-882.
- Zhao P, Zhang S, Liu L, Pang X, Yang Y, et al. (2018) Differences in the Triacylglycerol and Fatty Acid Compositions of Human Colostrum and Mature Milk. J Agric Food Chem6 6: 4571-4579.
- Linderborg KM, Kalpio M, Mäkelä J, Niinikoski H, Kallio HP, et al. (2014) Tandem mass spectrometric analysis of human milk triacylglycerols from normal weight and overweight mothers on different diets. Food Chem 146: 583-590.
- Zhang X, Qi C, Zhang Y, Wei W, Jin Q, et al. (2019) Identification and quantification of triacylglycerols in human milk fat using ultra-performance convergence chromatography and quadrupole time-of-flight mass spectrometery with supercritical carbon dioxide as a mobile phase. Food Chem 275: 712-720.
- Lindahl IEI, Artegoitia VM, Downey E, O’mahony JA, O’shea CA, et al. (2019) Quantification of Human Milk Phospholipids: the Effect of Gestational and Lactational Age on Phospholipid Composition. Nutrients 11: 222.
- Liu Z, Moate P, Cocks B, Rochfort S (2015) Comprehensive polar lipid identification and quantification in milk by liquid chromatography-mass spectrometry. J Chromatogr B AnalytTechnol Biomed Life Sci 978-979: 95-102.
- Cilla A, Quintaes KD, Barberá R, Alegría A (2016) Phospholipids in Human Milk and Infant Formulas: Benefits and Needs for Correct Infant Nutrition. Crit Rev Food SciNutr 56: 1880-1892.
- Selvalatchmanan J, Rukmini AV, Ji S, Triebl A, Gao L, et al. (2021) Variability of Lipids in Human Milk. Metabolites 11: 1-18.
- Brink LR, Lönnerdal B (2020) Milk fat globule membrane: the role of its various components in infant health and development. J Nutr Biochem 85: 108465.
- Chua ECP, Shui G, Lee ITG, Lau P, Tan LC, et al. (2013) Extensive diversity in circadian regulation of plasma lipids and evidence for different circadian metabolic phenotypes in humans. Proc Natl Acad Sci USA 110: 14468-14473.
- Donovan SM (2019) Human Milk Proteins: Composition and Physiological Significance. Nestle Nutrition Institute workshop series 90: 93-101.
- Lönnerdal B (2017) Bioactive Proteins in Human Milk-Potential Benefits for Preterm Infants. Clin Perinatol 44: 179-191.
- Lukacka MC, Pawilowicz MO, Broers B, Olejnik BK (2019)Lactoferrin in Human Milk of Prolonged Lactation. Nutrients 11: 2350.
- Hester SN, Hustead DS, MacKey AD, Singhal A, Marriage BJ (2012)Is the macronutrient intake of formula-fed infants greater than breast-fed infants in early infancy? J Nutr Metab 2012: 891201.
- Bullen JJ, Rogers HJ, Leigh L (1972) Iron-binding proteins in milk and resistance to Escherichia coli infection in infants. Br Med J 1: 69-75.
- Buccigrossi V, Marco G, Bruzzese E, Ombrato L, Bracale I, et al. (2007) Lactoferrin induces concentration-dependent functional modulation of intestinal proliferation and differentiation. Pediatr Res 61: 410-414.
- Layman DK, Lönnerdal B, Fernstrom JD (2018) Applications for α-lactalbumin in human nutrition. Nutr Rev 76: 444-460.
- Lönnerdal B (2016) Bioactive Proteins in Human Milk: Health, Nutrition, and Implications for Infant Formulas. J Pediatr 173: 4-9.
- Kelleher SL, Chatterton D, Nielsen K, Lönnerdal B (2003)Glycomacropeptide and alpha-lactalbumin supplementation of infant formula affects growth and nutritional status in infant rhesus monkeys. Am J ClinNutr 77: 1261-1268.
- Sandström O, Lönnerdal B, Graverholt G, Hernell O (2008) Effects of alpha-lactalbumin-enriched formula containing different concentrations of glycomacropeptide on infant nutrition. Am J Clin Nutr 87: 921-928.
- Chatterton DEW, Nguyen DN, Bering SB, Sangild PT (2013) Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int J Biochem Cell Biol 45: 1730-1747.
- Sun S, Liu F, Liu G, Miao J, Xiao H, et al. (2018) Effects of casein phosphopeptides on calcium absorption and metabolism bioactivity in vitro and in vivo. Food Funct 9: 5220-5229.
- Brink LR, Lönnerdal B (2022) Milk fat globule membrane: the role of its various components in infant health and development. J NutrBiochem 85: 108465.
- Liao Y, Alvarado R, Phinney B, Lönnerdal B (2011) Proteomic characterization of human milk fat globule membrane proteins during a 12 month lactation period. J Proteome Res 10: 3530-3541.
- Peterson JA, Patton S, Hamosh M (1998) Glycoproteins of the human milk fat globule in the protection of the breast-fed infant against infections. Biol Neonate 74:143-162.
- Imam A, Drushella MM, Taylor CR, Tökés ZA (1986) Preferential Expression of a Mr 155,000 Milk-Fat-Globule Membrane Glycoprotein on Luminal Epithelium of Lobules in Human Breast. Cancer Research 46: 6374-6379.
- Snow DR, Flores RJ, Ward RE, Cambell J, Young MJ, et al. (2010) Dietary milk fat globule membrane reduces the incidence of aberrant crypt foci in Fischer-344 rats. J Agric Food Chem 58: 2157-2163.
- Gridneva Z, Rea A, Tie WJ, Lai CT, Kugananthan S, et al. (2019) Carbohydrates in Human Milk and Body Composition of Term Infants during the First 12 Months of Lactation. Nutrients 11: 1472.
- Wicinski M, Sawicka E, Gebalski J, Kubiak K, Malinowski B (2020) Human Milk Oligosaccharides: Health Benefits, Potential Applications in Infant Formulas, and Pharmacology. Nutrients 12: 266.
- Walsh C, Lane JA, Sinderen D, Hickey RM (2020) Human milk oligosaccharides: Shaping the infant gut microbiota and supporting health. Journal of Functional Foods 72: 104074.
- Chong CYL, Bloomfield FH, O’Sullivan JM (2018) Factors Affecting Gastrointestinal Microbiome Development in Neonates. Nutrients 10: 274.
- Lyons KE, Ryan CA, Dempsey EM, Ross RP, Stanton C (2020) Breast Milk, a Source of Beneficial Microbes and Associated Benefits for Infant Health. Nutrients 12: 1039.
- Lagström H, Rautava S, Ollila H, Kaljonen A, Turta O, et al. (2020) Associations between human milk oligosaccharides and growth in infancy and early childhood. Am J ClinNutr 111: 769-778.
- Diaz AG, Arribas SM, Algara A, Cabrejas MAM, Pablo ÁLL, et al. (2019) A Review of Bioactive Factors in Human Breastmilk: A Focus on Prematurity. Nutrients 11: 1307.
- Fields DA, Demerath EW (2012) Relationship of insulin, glucose, leptin, IL-6 and TNF-α in human breast milk with infant growth and body composition. Pediatr Obes 7: 304-312.
- Alderete TL, Autran C, Brekke BE, Knight R, Bode L, et al. (2015) Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am J Clin Nutr 102: 1381-1388.
- Chan D, Goruk S, Becker AB, Subbarao P, Mandhane PJ, et al. (2018) Adiponectin, leptin and insulin in breast milk: associations with maternal characteristics and infant body composition in the first year of life. Int J Obes (Lond) 42: 36-43.
- Khodabakhshi A, Mobarhan MG, Rooki H, Vakili R, Hashemy SI, et al. (2015) Comparative measurement of ghrelin, leptin, adiponectin, EGF and IGF-1 in breast milk of mothers with overweight/obese and normal-weight infants. Eur J Clin Nutr 69: 614-618.
- Galante L, Pundir S, Lagström H, Rautava S, Reynolds CM, et al. (2020) Growth Factor Concentrations in Human Milk Are Associated With Infant Weight and BMI From Birth to 5 Years. Front Nutr 7: 110.
- Logan CA, Siziba LP, Koenig W, Carr P, Brenner H, et al. (2019) Leptin in Human Milk and Child Body Mass Index: Results of the Ulm Birth Cohort Studies. Nutrients 11: 1883.
- Uysal FK, Önal EE, Aral YZ, Adam B, Dilmen U, et al. (2002) Breast milk leptin: its relationship to maternal and infant adiposity. Clin Nutr 21: 157-160.
- Mazzocchi A, Giannì ML, Morniroli D, Leone L, Roggero P, et al. (2019) Hormones in Breast Milk and Effect on Infants’ Growth: A Systematic Review. Nutrients 11: 1845.
- Kon IY, Shilina NM, Gmoshinskaya MV, Ivanushkina TA (2014) The study of breast milk IGF-1, leptin, ghrelin and adiponectin levels as possible reasons of high weight gain in breast-fed infants. Ann Nutr Metab 65: 317-323.
- IoM (Institute of Medicine) (2015). Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. National Academies Press (Pg No’s 1-617), Washington DC, USA.
- Dórea JG (200) Magnesium in human milk. J Am Coll Nutr 19: 210-219.
- Rodriguez EMR, Alaejos MS, Romero CD (2000) Concentrations of iron, copper and zinc in human milk and powdered infant formula. Int J Food Sci Nutr 51: 373-380.
- Olausson H, Goldberg GR, Laskey MA, Schoenmakers I, Jarjou LMA, et al. (2012) Calcium economy in human pregnancy and lactation. Nutr Res Rev 25: 40-67.
- Corkins GK, Shurley T (2016) What’s in the Bottle? A Review of Infant Formulas. Nutr Clin Pract 31: 723-729.
- Prell C, Koletzko B (2016) Breastfeeding and Complementary Feeding. Dtsch Arztebl Int 113: 435-444.
- Zou L, Pande G, Akoh CC (2016) Infant Formula Fat Analogs and Human Milk Fat: New Focus on Infant Developmental Needs. Annu Rev Food Sci Technol 7: 139-165.
- Ahern GJ, Hennessy AA, Anthony Ryan C, Paul Ross R, Stanton C (2019) Advances in Infant Formula Science. Annu Rev Food Sci Technol 10: 75-102.
- Owens CJW, Labuschagne IL, Lombard MJ (2014) The basics of prescribing infant formulas. South African Family Practice 54: 25-30.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (2014) Scientific Opinion on the essential composition of infant and follow-on formulae. EFSA 12: 3760.
- Thompkinson DK, Kharb S (2007) Aspects of Infant Food Formulation. Comprehensive Reviews in Food Science and Food Safety 6: 79-102.
- Mazzocchi A, D’Oria V, de Cosmi V, Bettocchi S, Milani GP, et al. (2018) The Role of Lipids in Human Milk and Infant Formulae. Nutrients 10: 567.
- Henriksen C, Haugholt K, Lindgren M, Aurvåg AK, Rønnestad A, et al. (2008) Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics 121: 1137-1145.
- Koletzko B, Baker S, Cleghorn G, Neto UF, Gopalan S, et al. (2005) Global standard for the composition of infant formula: recommendations of an ESPGHAN coordinated international expert group. J Pediatr Gastroenterol Nutr 41: 584-599.
- Timby N, Hernell O, Vaarala O, Melin M, Lönnerdal B, et al. (2015) Infections in infants fed formula supplemented with bovine milk fat globule membranes. J Pediatr Gastroenterol Nutr 60: 384-389.
- Fanaro S, Ballardini E, Vigi V (2010) Different pre-term formulas for different pre-term infants. Early Hum Dev 86: 27-31.
- Lönnerdal B (2014) Infant formula and infant nutrition: bioactive proteins of human milk and implications for composition of infant formulas. Am J Clin Nutr 99: 712S-717S.
- Deglaire A, de Oliveira SC, Jardin J, Briard-Bion V, Emily M, et al. (2016) Impact of human milk pasteurization on the kinetics of peptide release during in vitro dynamic term newborn digestion. Electrophoresis 37: 1839-1850.
- Koletzko B, von Kries R, Closa R, Escribano J, Scaglioni S, et al. (2009) Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr 89: 1836-1845.
- Weber M, Grote V, Closa-Monasterolo R, Escribano J, Langhendries JP, et al. (2014) Lower protein content in infant formula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am J Clin Nutr 99: 1041-1051.
- Vandenplas Y, de Greef E, Veereman G (2014) Prebiotics in infant formula. Gut Microbes 5: 681-687.
- Gibson GR, Roberfroid MB (1995) Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 125: 1401-1412.
- Vandenplas Y, Zakharova I, Dmitrieva Y (2015) Oligosaccharides in infant formula: more evidence to validate the role of prebiotics. Br J Nutr 113: 1339-1344.
- Bettler J, Euler A, Nutrition W (2006) An evaluation of the growth of term infants fed formula supplemented with fructo-oligosaccharide. Int J Probiotics Prebiotics 1: 19-26.
- Grüber C, van Stuijvenberg M, Mosca F, Moro G, Chirico G, et al. (2010) Reduced occurrence of early atopic dermatitis because of immunoactive prebiotics among low-atopy-risk infants. J Allergy Clin Immunol 126: 791-797.
Citation: Garro-Aguilar Y, Gulak M, Astigarraga E, Barreda-Gómez G (2022) Breastfeeding: History, Techniques, Benefits, Complications and Care. J Pract Prof Nurs 6: 031.
Copyright: © 2022 Yaiza Garro-Aguilar, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

