
Evaluation of Different Treatment Modalities on Survival of 135 Patients with Low-Grade Endometrial Stromal Sarcoma
*Corresponding Author(s):
Yan HuangDepartment Of Oncology, Shanghai Medical College, Fudan University, 270 Dong-an Road, Shanghai 200032, China
Tel:+86 216417559082900,
Fax:+86 2164220677
Email:huangyan1168@aliyun.com
Abstract
Background: To evaluate the influence of different treatment modalities on survival of patients with Low-Grade Endometrial Stromal Sarcoma (LGESS).
Methods: One hundred and thirty-five LGESS patients in Fudan University Shanghai Cancer Center from January 2006 to December 2018 were retrospectively reviewed.
Results: The median follow-up duration was 52 months (3-342 months). Two patients received fertility-sparing surgery while 133 patients received hysterectomy. One hundred and nine (80.7%) patients received ovariectomy, 73 (54.1%) patients had lymphadenectomy and 83 (61.5%) patients received adjuvant therapy. The 5-year and 10-year disease free survival rates were 72.0% and 61.0%, respectively. The 5-year and 10-year overall survival rates were 88.0% and 79.8%, respectively. Surgery for recurrence was associated with improved overall survival (p<0.05) although the complication rate was about 10.3%. Multivariate analyses showed that lymphovascular invasion was associated with disease free survival (hazard ratio, 0.473; 95% confidence interval, 0.235-0.952; p=0.036) and menopausal status was related to overall survival (hazard ratio, 5.561; 95% confidence interval, 1.400-22.084; p=0.015).
Conclusion: Hysterectomy may be proposed as the standard treatment for LGESS. Surgery for replase was an acceptable method to improve overall survival.
Keywords
Low-grade endometrial stromal sarcoma; Prognosis; Treatment
LIST OF ABBREVIATIONS
ESS: Endometrial stromal sarcoma
UUS: Undifferentiated Uterine Sarcoma
LGESS: Low-Grade Endometrial Stromal Sarcoma
HGESS: High-Grade Endometrial Stromal Sarcoma
WHO: World Health Organization
FIGO: International Federation of Gynecology and Obstetrics
CT: Computed Tomography
MRI: Magnetic Resonance Imaging
PET-CT: Positron Emission Tomography-Computed Tomography
SPSS: Statistic Package for Social Science
BACKGROUND
Uterine sarcomas represent 8% of all uterine malignancies and Endometrial Stromal Sarcoma (ESS) accounts for approximately 20% of all uterine sarcomas [1]. There are 2 categories of ESS: Low-Grade Endometrial Stromal Sarcoma (LGESS), and High-Grade Endometrial Stromal Sarcoma (HGESS) according to the 2014 World Health Organization (WHO) Classification of Tumors [2-4]. LGESS is the most common type of ESS, and hysterectomy is regarded as the main treatment [5,6]. However, the roles of other therapies such as ovariectomy, lymphadenectomy, and postoperative adjuvant therapy, remain controversial [7-9]. In the present study, we retrospectively reviewed the treatments given to patients with LGESS to evaluate the efficacy of different treatment modalities on survival. The associations between clinicopathologic variables and survival were also evaluated.
METHODS
The Review Board of Fudan University Shanghai Cancer Center approved the study. The study included the LGESS patients from January 2006 to December 2018 who had been pathologically diagnosed according to 2014 WHO classification and staged based on International Federation of Gynecology and Obstetrics (FIGO) 2009 staging classification. The pathology was conducted by experienced pathologists of our hospital (cases from other hospitals were all taken for consultation). Two patients who wanted to preserve the fertility received hysteromyoma. The surgery generally included total hysterectomy ± bilateral salpingo-oophorectomy ± lymphadenectomy. For patients with multiple metastases, cytoreductive surgeries were performed. Hormone therapy comprised Megestrol acetate 160 mg/d or Letrozole 2.5mg/d or Medroxyprogesterone acetate 500 mg/d. The radiation treatment included external pelvic irradiation (18 MV X-rays) with one fraction of 1.8-2.0 Gy daily for a total dose of 50 Gy in 5-6 weeks. Chemotherapy comprised one of the following combinations for 3-6 cycles: cisplatin 50 mg/m2 (d1) + cyclophosphamide 500 mg/m2 (d1) + epirubicin 60 mg/m2 (d1), or gemcitabine 900 mg/m2 (d1, 8) + docetaxel 100 mg/m2 (d8), or doxorubicin 60 mg/m2 (d1) + ifosfamide 1.5 g/m2 (d1-4).
Patient clinicopathologic factors included age, menopausal status, patient’s symptom, laparoscopic myomectomy, tumor size, muscular infiltration, lymphovascular invasion, lymph node metastasis, FIGO stage, lymphadenectomy, ovariectomy, adjuvant therapy, residual disease and recurrence. When the recurrence was suspected, the patients would be examined by needle biopsy, or Computed Tomography (CT), or Magnetic Resonance Imaging (MRI), or Positron Emission Tomography-Computed Tomography (PET-CT) on the basis of physical examination. The median follow-up duration was 52 months (3-342 months). The overall survival was calculated as the months from the date of the first surgery to either the date of last follow up or the date of death. The disease free survival was calculated as the months from the date of the first surgery to either the date of last follow up or the date of the first recurrence.
All statistical analyses were done with Statistic Package for Social Science (SPSS) version 16.0 (Incorporated, Chicago, Illinois, the United States). The disease free survival and overall survival were analyzed by the Kaplan-Meier method, and compared using the log rank test. Univariate and multivariate Cox regression analyses were performed to analyze prognostic factors. A p-value less than 0.05 were considered statistically significant.
RESULTS
Clinicopathologic factors and treatment modalities of LEGSS patients
A total of 135 LGESS patients were retrospectively analyzed. Clinicopathological characteristics were presented in table 1. Median age at diagnosis was 41.2 years (19-65 years). One hundred and eighteen patients (87.4%) were premenopausal. Most (62.2%) patients had no symptoms and the second symptom was abnormal vaginal bleeding in 37 (27.4%) patients. Thirty (22.2%) patients had the history of laparoscopic myomectomy and 56 (41.5%) patients received a secondary operation after the first surgery was hysteromyoma or subtotal hysterectomy.
|
Characteristics |
n (%) |
|
|
Age (years) |
||
|
|
Median (range) |
41.2 (19-65) |
|
|
≤50 |
112 (83.0%) |
|
|
>50 |
23 (17.0%) |
|
Menopausal status |
||
|
|
Premenopausal |
118 (87.4%) |
|
|
Postmenopausal |
17 (12.6%) |
|
Patient’s symptom |
||
|
|
No |
84 (62.2%) |
|
|
Abnormal vaginal bleeding |
37 (27.4%) |
|
|
Abdominal pain |
12 (8.9%) |
|
|
Others |
2 (1.5%) |
|
Laparoscopic myomectomy |
||
|
|
Yes |
30 (22.2%) |
|
|
No |
105 (77.8%) |
|
Tumor size |
||
|
|
≤5cm |
39 (28.9%) |
|
|
>5cm |
96 (71.1%) |
|
Muscular infiltration |
||
|
|
≤1/2 |
32 (23.7%) |
|
|
>1/2 |
103 (76.3%) |
|
Lymphovascular invasion |
||
|
|
Yes |
33 (24.4%) |
|
|
No |
102 (75.6) |
|
Lymph node metastasis |
||
|
|
Yes |
9 (6.7%) |
|
|
No |
126 (92.6%) |
|
FIGO stage |
||
|
|
I |
108 (80.0%) |
|
|
II |
13 (9.6%) |
|
|
III |
12 (8.9%) |
|
|
IV |
2 (1.5%) |
Table 1: Clinicopathological characteristics of LGESS patients (n=135).
Large tumor size (≥5 cm) was found in 96 (71.1%) cases. Deep muscle infiltration was observed in 103 (76.3%) patients. Positive lymphovascular invasion was in 33 (24.4%) patients. Lymph node metastasis was shown in 9 (6.7%) patients. FIGO staging indicated that 108 patients (80.0%) had stage I, 13 (9.6%) had stage II, 12 (8.9%) had stage III, and 2 (1.5%) had stage IV. Table 2 summarized the different treatments and outcomes in 135 LGESS patients. Two patients received fertility-sparing surgery and 133 patients received hysterectomy. One hundred and nine (80.7%) patients received ovariectomy and 73 (54.1%) patients had lymphadenectomy. Fifty-two (38.5%) patients had no adjuvant treatment, 38 (28.1%) patients received hornome therapy, 22 (16.3%) patients had radiotherapy and hornome therapy, and 23 (17.0%) patients received chemotherapy and hornome therapy. Ten (7.4%) patients had residual disease after surgery.
|
Characteristics |
n (%) |
|
|
Hysterectomy |
||
|
|
Yes |
133 (98.5%) |
|
|
No |
2 (1.5%) |
|
Lymphadenectomy |
||
|
|
Yes |
73 (54.1%) |
|
|
No |
62 (45.9%) |
|
Ovariectomy |
||
|
|
Yes |
109 (80.7%) |
|
|
No |
26 (19.3%) |
|
Adjuvant therapy |
||
|
|
No |
52 (38.5%) |
|
|
Hormonal therapy |
38 (28.1%) |
|
|
Radiotherapy and hormonal therapy |
22 (16.3%) |
|
|
Chemotherapy and hormonal therapy |
23 (17.0%) |
|
Residual disease |
||
|
|
Yes |
10 (7.4%) |
|
|
No |
125 (93.3%) |
|
Recurrence |
||
|
|
No |
96 (71.1%) |
|
|
Local pelvic recurrence |
30 (22.2%) |
|
|
Others |
9 (6.7%) |
|
Death |
||
|
|
Yes |
17 (12.6%) |
|
|
No |
118 (87.4%) |
Table 2: Different treatments and outcomes in patients with LGESS (n=135).
Survival and recurrence
The patients were followed up for a median duration of 52 months (3-342 months). The 5-year and 10-year disease free survival rates were 72.0% and 61.0%, respectively (Figure 1A). The 5-year and 10-year overall survival rates were 88.0% and 79.8%, respectively (Figure 1B). Thirty-nine (28.9%) patients had disease recurrence, with a median time to recurrence of 24 months (1-321 months). The distribution of recurrences according to the stage of disease was as follows: 29 out of 108 (26.9%) patients were stage I, 5 out of 13 (38.5%) stage II and 5 out of 12 (41.7%) stage III. Pelvis was the main recurrent site in 76.9% (30 of 39) patients. The other recurrent sites included intestine (n=4), omentum (n=1), liver (n=2), and lung (n=2). The median survival after recurrence was 17 months (1-177 months). Twenty-nine patients received cytoreductive surgery for recurrences. It was associated with improved mean survival of 47.5 months as compared to mean survival of 14.8 months in 10 patients without it. So the surgery was related to improved overall survival (p<0.05, Figure 2). The main surgical complications for recurrent diseases were as follows: intestinal fistula (n=2), and urinary fistula (n=1). The complication rate was 10.3%. At the time of last follow up, 17 patients had died of cancer-related diseases.
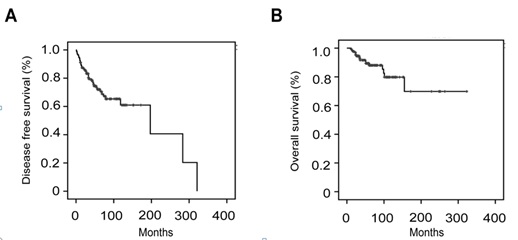 Figure 1: Disease free survival (A) and overall survival (B) in LGESS.
Figure 1: Disease free survival (A) and overall survival (B) in LGESS.
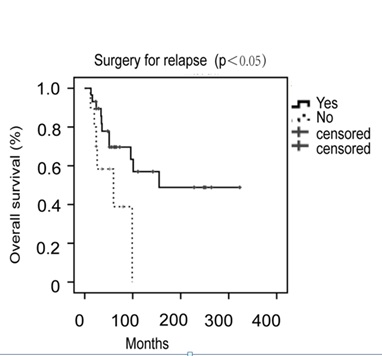 Figure 2: Overall survival by cytoreductive surgery for relapse in LGESS.
Figure 2: Overall survival by cytoreductive surgery for relapse in LGESS.
Prognostic factors associated with disease free survival or overall survival
In univariate analyses for disease free survival, menopausal status was associated with disease free survival (p<0.05). So were deep muscle infiltration (p<0.05) and lymphovascular invasion (p<0.01). However, upon multivariate analyses, only lymphovascular invasion remained as an independent predictor of disease free survival (hazard ratio, 2.062; 95 % confidence interval, 1.040-4.086; p=0.038) (Figure 3A). In univariate analyses for overall survival, menopausal status was associated with overall survival (p<0.01). So were FIGO stage (p<0.05), lymphovascular invasion (p<0.05), lymph node metastasis (p<0.05), residual disease (p<0.05) and recurrence (p<0.01). However, upon multivariate analyses, only menopausal status remained as an independent predictor of overall survival (hazard ratio, 3.691; 95 % confidence interval, 1.012-13.457; p=0.048) (Figure 3B). When we further assessed different treatment methods and prognotic factors in 108 LGESS patients with stage I, we still found the similar results: lymphovascular invasion was associated with disease free survival and menopausal status was related to overall survival at multivariate analyses.
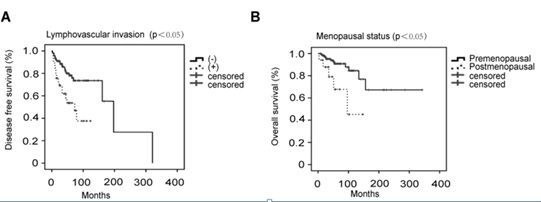 Figure 3: Disease free survival by lymphovascular invasion (A) and overall survival by menopausal status (B).
Figure 3: Disease free survival by lymphovascular invasion (A) and overall survival by menopausal status (B).
DISCUSSION
Endometrial stromal sarcomas were rare uterine malignancies that might manifest through abnormal uterine bleeding and pelvic mass [6]. In our study, the majority (62.2%) of LGESS patients had no symptoms and the second symptom was abnormal vaginal bleeding in 37(27.4%) patients (Table 1). Because the preoperative diagnosis was ambiguous and the intraoperative frozen pathology had its limitation, almost all the patients were diagnosed postoperatively. Thus, 56 (41.5%) patients received a secondary operation after the first surgery was hysteromyoma or subtotal hysterectomy. Moreover, laparoscopy was often used in the first operation. Choo suggested that intrapelvic dissemination was due to electronic morcellation [10]. A consensus review suggested morcellation should be avoided [1]. However, we found the history of laparoscopic myomectomy was related to neither overall survival nor disease free survival.
The mean age at diagnosis was 41.2 years (19-65 years) and 118 patients (87.4%) were premenopausal. Therefore, it was worth considering fertility-sparing surgery or ovarian preservation. Zhou suggested that ovarian preservation had no significant effect on disease free survival and ovarian preservation was feasible [6]. It was also reported that fertility-sparing surgery may be considered for early-stage LGESS patients [11,12]. Rather, some study suggested that the removal of the adnexa might be helpful to decrease the risk of recurrence [13]. In our study, 2 patients received fertility-sparing surgery with postoperative hormone therapy and no recurrences occured yet. Moreover, our analysis showed that ovarian removal had no significant effect on disease free survival (p=0.443) and overall survival (p=0.854).
According to 2009 FIGO guidelines, initial complete staging for endometrial stromal sarcoma would need lymphadenectomy. Previous studies demostrated that the incidence of lymph node metastasis ranged from 16% to 33% [14,15]. In our study, 73 (54.1%) patients received lymphadenectomy and only 9 (6.7%) cases had lymph node metastases. Currently, the benefit of lymph node resection in LGESS is controversial. One meta-analysis suggests that lymphadenectomy bore little prognostic or therapeutic benefit in patients with uterine sarcoma [16]. However, another study showed total hysterectomy and bilateral salpingo-oophorectomy followed by pelvic lymphadenectomy was associated with an improved outcome [17]. Our data found that lymphadenectomy had no effect on both disease free survival (p=0.246) and overall survival (p=0.652). So, we did not advocate lymphadenectomy in LGESS patients without lymphatic metastasis.
The ability of adjuvant treatment in patients with uterine sarcomas was unclear and there was no standard recommendation regarding adjuvant therapy [18]. Schick found that adjuvant radiotherapy was an independent prognostic factor for overall survival [19]. Use of adjuvant chemotherapy and radiotherapy were reported to be associated with better prognosis only for HGESS [9,20]. Cade’s study did not regard primary adjuvant progestogen as a survival benefit [21]. Although most previous articles recommended postoperative hormone therapy, our paper did not find hormone therapy was associated with survival due to limited data. The recurrent rate increased with the development of the stage in our research. The median time to recurrence was 24 months (1-321 months). Twenty-nine (74.4%) out of 39 recurrent LGESS patients received cytoredductive surgery. The surgery for recurrence was associated with improved overall survival (p<0.05) although the complication rate was about 10.3%. Yamazaki also found that the post-relapse survival of patients with endometrial stromal sarcoma can be expected to be >10 years when treated by repeated surgical resection [22]. So repeated surgery for recurrent disease should be an acceptable method. Preoperative intestinal and urinary preparation may help to reduce the rate of the complications.
Bai reported that the 5-year relapse free survival and overall survival rates were 66.1% and 95.8% in LGESS [23]. In our study, the 5-year disease free survival and overall survival rates were 72.0% and 88.0% and the 10-year disease free survival and overall survival rates were 61.0% and 79.8%. Khatib found that stage, age, lymphovascular invasion, and lymphadenectomy were independent prognostic factors for disease free survival and so was stage for overall survival [24]. Another paper showed that age, lymphadenectomy, stage I, and adjuvant therapy did not affect disease free survival or overall survival [25]. On multivariate analyses, only lymphovascular invasion was an independent predictor for disease free survival and so was menopausal status for overall survival in our research. So we suggested lymphovascular invasion was a high risk factor for recurrence and uterine tumors after menopause are well worth our attention.
CONCLUSION
In conclusion, hysterectomy may be proposed as the standard treatment. Cytoreductive surgery for relapse could improve overall survival in recurrent cases. Lymphovascular invasion was a significant independent factor for disease free survival. Post-menopause was the poor prognostic factor for overall survival.
DECLARATIONS
Ethics approval and consent to participate
This study was conducted according to the declaration of Helsinki and was approved by the Committee of Fudan University Shanghai Cancer Center.
Consent for publication
Written informed consents were obtained from all individual participants included in the study.
Availability of data and materials
The institutional database involves sensitive patient information, which is available upon request. Anyone who is interested in the information should contact huangyan1168@aliyun.com.
COMPETING INTERESTS
The authors declare that they have no competing interests.
FUNDING
This work was supported by Science and Technology Commission of Shanghai Municipalicy (grant no. 11ZR1407700) of Yan Huang and National Natural Science Foundation of China (grant no. 81202051) of Yan Huang.
AUTHORS’ CONTRIBUTIONS
Yan Huang participated in the study design, carried out the data collection, performed the statistical analysis, and revised the manuscript. Huaying Wang carried out the data collection, performed the statistical analysis and drafted the manuscript. Shanhui Liang and Zheng Feng carried out the data collection. Jun Zhu and Lingfang Xia conceived of the study, and participated in its design and coordination. All authors read and approved the final manuscript.
ACKNOWLEDGEMENT
We would like to thank all the doctors, nurses, patients, and their family members for their kindness to support our study.
REFERENCES
- Amant F, Floquet A, Friedlander M, Kristensen G, Mahner S, et al. (2014) Gynecologic Cancer InterGroup (GCIG) consensus review for endometrial stromal sarcoma. Int J Gynecol Cancer 24: 67-72.
- Ali RH, Rouzbahman M (2015) Endometrial stromal tumours revisited: An update based on the 2014 WHO classification. J Clin Pathol 68: 325-332.
- Conklin CM, Longacre TA (2014) Endometrial stromal tumors: The new WHO classification. Adv Anat Pathol 21: 383-393.
- Horng HC, Wen KC, Wang PH, Chen YJ, Yen MS, et al. (2016) Uterine sarcoma Part II-Uterine endometrial stromal sarcoma: The TAG systematic review. Taiwan J Obstet Gynecol 55: 472-479.
- Feng W, Hua K, Malpica A, Zhou X, Baak JP (2013) Stages I to II WHO 2003-defined low-grade endometrial stromal sarcoma: How much primary therapy is needed and how little is enough? Int J Gynecol Cancer 23: 488-493.
- Zhou J, Zheng H, Wu SG, He ZY, Li FY, et al. (2015) Influence of different treatment modalities on survival of patients with low-grade endometrial stromal sarcoma: A retrospective cohort study. Int J Surg 23: 147-151.
- Reich O, Regauer S, Urdl W, Lahousen M, Winter R (2000) Expression of oestrogen and progesterone receptors in low-grade endometrial stromal sarcomas. Br J Cancer 82: 1030-1034.
- Rauh-Hain JA, del Carmen MG (2013) Endometrial stromal sarcoma: A systematic review. Obstet Gynecol 122: 676-683.
- Seagle BL, Shilpi A, Buchanan S, Goodman C, Shahabi S (2017) Low-grade and high-grade endometrial stromal sarcoma: A National Cancer Database study. Gynecol Oncol 146: 254-262.
- Choo KJ, Lee HJ, Lee TS, Kim JH, Koh SB, et al. (2015) Intrapelvic dissemination of early low-grade endometrioid stromal sarcoma due to electronic morcellation. Obstet Gynecol Sci 58: 414-417.
- Xie W, Cao D, Yang J, Jiang X, Shen K, et al. (2017) Fertility-sparing surgery for patients with low-grade endometrial stromal sarcoma. Oncotarget 8: 10602-10608.
- Jin Y, Li Y, Deng CY, Tian QJ, Chen H, et al. (2015) Fertility-sparing treatment of low-grade endometrial stromal sarcoma. Int J Clin Exp Med 8: 5818-5821.
- Beck TL, Singhal PK, Ehrenberg HM, Rose PG, Lele SB, et al. (2012) Endometrial stromal sarcoma: Analysis of recurrence following adjuvant treatment. Gynecol Oncol 125: 141-144.
- Signorelli M, Fruscio R, Dell'Anna T, Buda A, Giuliani D, et al. (2010) Lymphadenectomy in uterine low-grade endometrial stromal sarcoma: An analysis of 19 cases and a literature review. Int J Gynecol Cancer 20: 1363-1366.
- Riopel J, Plante M, Renaud MC, Roy M, Têtu B (2005) Lymph node metastases in low-grade endometrial stromal sarcoma. Gynecol Oncol 96: 402-406.
- Si M, Jia L, Song K, Zhang Q, Kong B (2017) Role of Lymphadenectomy for Uterine Sarcoma: A Meta-Analysis. Int J Gynecol Cancer 27: 109-116.
- He L, Li JD, Xiong Y, Huang X, Huang L, et al. (2014) Clinicopathological and molecular markers associated with prognosis and treatment effectiveness of endometrial stromal sarcoma: A retrospective study in China. Arch Gynecol Obstet 289: 383-391.
- Chern JY, Boyd LR, Blank SV (2017) Uterine Sarcomas: The Latest Approaches for These Rare but Potentially Deadly Tumors. Oncology (Williston Park) 31: 229-236.
- Schick U, Bolukbasi Y, Thariat J, Abdah-Bortnyak R, Kuten A, et al. (2012) Outcome and prognostic factors in endometrial stromal tumors: A Rare Cancer Network study. Int J Radiat Oncol Biol Phys 82: 757-763.
- Agarwal R, Rajanbabu A, Nair IR, Satish C, Jose G, et al. (2017) Endometrial stromal sarcoma-A retropsective analysis of factors affecting recurrence. Eur J Obstet Gynecol Reprod Biol 216: 92-97.
- Cade TJ, Quinn MA, Rome RM, Polyakov A (2014) Prognostic significance of steroid receptor positivity and adjuvant progestogen use in endometrial stromal sarcoma. Aust NZJ Obstet Gynaecol 54: 453-456.
- Yamazaki H, Todo Y, Mitsube K, Hareyama H, Shimada C, et al. (2015) Long-term survival of patients with recurrent endometrial stromal sarcoma: A multicenter, observational study. J Gynecol Oncol 26: 214-221.
- Bai H, Yang J, Cao D, Huang H, Xiang Y, et al. (2014) Ovary and uterus-sparing procedures for low-grade endometrial stromal sarcoma: A retrospective study of 153 cases. Gynecol Oncol 132: 654-660.
- Khatib G, Guzel AB, Gulec UK, Gumurdulu D, Vardar MA, et al. (2014) Clinicopathological features and prognostic factors of the uterine sarcomas: 20 years of experience at Cukurova University. Eur J Gynaecol Oncol 35: 646-654.
- Rauh-Hain JA, Goodman A, Boruta DM, Schorge JO, Horowitz NS, et al. (2014) Endometrial stromal sarcoma: a clinicopathologic study of 29 patients. J Reprod Med 59: 547-552.
Citation: Wang H, Liang S, Feng Z, Xia L, Zhu J, et al. (2020) Evaluation of Different Treatment Modalities on Survival of 135 Patients with Low-Grade Endometrial Stromal Sarcoma. J Reprod Med Gynecol Obstet 5: 045.
Copyright: © 2020 Huaying Wang, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

