
High Iodine Status in the Hair Samples of Well-Nourished Thai Children
*Corresponding Author(s):
Torsak TippairoteNutritional And Environmental Medicine Section, Healing Passion Medical Center, Bangkok, Thailand
Tel:+66 818584975,
Email:maeklong@yahoo.com / torsak@healingpassion-asia.com
Abstract
Background: Thailand is a country with adequate iodine intake status. There were increasing reports of excessive iodine exposure in a specific children group, which could also elicit adverse health consequences.
Objectives: This study explored the hair iodine levels of well-nourished childrenfrom Bangkok and the perimeters. The findings of a broad randge, high average level, and extreme outliners warranted the risk assessment procedures and the awareness-raising of excessive iodine exposure consequences in a specific children group.
Methods: We performed the secondary analysis of the dataset from a case-control study of Thai children's hair element levels in the study group with Attention Deficit and Hyperactive Disorders (ADHD) and the healthy control group. We compared the differences in hair iodine levels with their age, sex, address, attending school types, religious, ADHD, and nutritional category status. We correlated these results with the previous national surveys of median Urine Iodine Concentration (UIC) levels in Thai Children.
Results: Children’s hair iodine levels had a broad range, from 0.81 to 28.00μg/gm, with a higher average level (3.07 ± 3.33μg/gm) than the previously documented values. The frequency distribution of participants in different quartiles was even. Several high outliners had their hair iodine levels between 7.4 to 28μg/gm. Children who lived in Bangkok and studied in private schools had higher hair iodine levels than their counterparts. Previous national children’s health surveys also reported the wide ranges and high median UIC levels in young children. These findings potentially suggested high iodine exposure in a specific group of Thai children.
Discussion: The double-edged iodine status in Thai children, from the insufficient intake to excessive exposure, was evident. While iodine deficiency awareness dominated public policy, there was an imminent risk of excessive iodine exposure in the group of young and well-nourished children from the middle- to high-income families in Bangkok.
Keywords
Children’s diet; Hair iodine; Iodine excess; Iodine status; Obese children
Introduction
Iodine is an essential trace mineral for children's health because of its crucial role in thyroid hormone synthesis. Iodine deficiency is a well-recognized global concernfor childhood brain and growth development and the worldwide, most common, preventable cause of mental retardation [1]. The worldwide campaign against iodine deficiency in school-age children was quite successful. According to the 2019 global scorecard of the iodine nutrition by the Iodine Global Network (IGN), 149 countries, or 86% of all 174 participating nations, had sufficient iodine intake status. These countries managed to keep their national survey levels of median Urine Iodine Concentration (UIC) above the WHO's criteria for insufficient intake limit at 100μg/L. However, 14 countries reported their median UIC levels above 300μg/L, which suggestedexcessive iodine intake status [2-4].
Excessive iodine exposure could elicit adverse health consequences comparable to insufficient iodine intake [5]. There were worldwide reports of potential sequelae from excessive iodine intake, which included a wide range of either transient or permanent thyroid dysfunction such as hypothyroidism, thyrotoxicosis, goiter, thyroid nodules, autoimmune thyroiditis, or the increased risk of specific thyroid cancer [6-16]. Despite these known facts, the awareness of potential consequences from excessive iodine intake isgenerally dormant.
We herein did the secondary analysis on a pilot case-control study dataset, which was previously published [17,18]. We explored the hair iodine levels of this study's participants and correlated these findings to the previous Thailand population surveys of median UIC levels in the different children-age groups. We then proposed an imminent risk of excessive iodine intake in a specific group of well-nourished Bangkok children from the family with middle to high socioeconomic status.
Materials and Methods
This case-control study compared the thirty-nine bio-element levels in hair samples of diagnosed Attention Deficit and Hyperactive Disorder (ADHD) children and their healthy controls from December 2015 to June 2016. With the known technical limitations of hair mineral analysis, there were the meticulous arrangements of sample collection, processing, and analytical processing through a certified U.S. laboratory service, as detailed in the previous reports [18,19]. The inductively coupled plasma mass spectrometry (ICP-MS) yielded the iodine level as micrograms per gram (μg/gm), or part per million, of dry hair weight.
Participants
The participants were one hundred and eleven Thai children, with the age range of three to seven years old. There were seventy-one boys and forty girls. Most of them lived in Bangkok (75%) and studied in private schools (77%), which indicated their families' socioeconomic status that ranged from middle to high levels. Almost all of them (95.6%) had the nutritional status categories as either healthy (58.2%), overweight (13.2%), or obese (24.4%), according to the Body-mass-index-for-AgeZ-scores (BAZ) following either the WHO growth standards for 0-5-year-old children [20], or the WHO growth references for over-5-year-old children [21].
Data processing and statistical analysis
We performed the dataset analysis through the Microsoft office365 spreadsheet (Microsoft Corp. Release 2016, Redmond, WA, USA) and IBM SPSS Statistics for Windows (IBM Corp. Release 2015, Version 23.0, Armonk, NY, USA). We processed all the statistical analyses by the non-parametric testing as the Kolmogorov-Smirnov normality tests did not show a normal distribution of the dataset despite the data transformation.
We derived the mean, median, minimum, maximum, and standard deviations of the hair iodine levels. We analyzed the average hair iodine levels against the different age, sex, and nutritional categories groups, either by the Mann-Whitney U test or the Kruskal Wallis Test. The Chi-Square testings were used for assessing the differences of the observed participant frequencies among the four quartiles of hair iodine levels. All testings used P-value at less than 0.05 as the limit for statistical significance.
To derive the cut-off level of hair iodine level in this dataset, we used the binary outcomes of age, sex, and nutritional status to evaluate through a receiver operating characteristic curve, or ROC. However, we did not get any practical cut-off level of hair iodine in this dataset.
Results
In tables 1 and 2 and figure 1, the average hair iodine levels in girls who lived in Bangkok and attended the private-schools were significantly higher than the boys, the suburban residents, and their public-school-attending counter parts. While the average hair iodine level of all participants was 3.1ug/gm, the well-nourished children, with healthy body weight, overweight, or obese, could present with the exceptional high hair iodine levels that ranged from 7.4 to 28ug/gm. These findings suggested the co-existing of the insufficient, adequate, and excessive iodine exposure among these children. The children group prone to excessive iodine exposure were those girls from middle and high socioeconomic families in Bangkok.
|
|
Hair Iodine level (μg/gm) |
|||||
|
N |
Mean |
SD |
Maximum |
P-value |
||
|
Total |
111 |
3.1 |
3.3 |
28.0 |
||
|
Younger than 5-year-olds |
45 |
3.5 |
4.5 |
28.0 |
0.496a |
|
|
Older than 5-year-olds |
66 |
2.7 |
2.2 |
12.0 |
||
|
Girls |
40 |
4.0 |
4.6 |
28.0 |
0.002a, * |
|
|
Boys |
71 |
2.5 |
2.2 |
12.0 |
||
|
Lived in Bangkok |
83 |
3.4 |
3.7 |
28.0 |
0.003a, * |
|
|
Lived in the Perimeters |
28 |
2.1 |
1.5 |
8.0 |
||
|
Private School |
85 |
3.3 |
3.6 |
28.0 |
0.011a, * |
|
|
Public School |
26 |
2.3 |
2.2 |
12.0 |
||
|
Buddhist |
99 |
3.2 |
3.5 |
28.0 |
0.082a |
|
|
Non-Buddhist |
12 |
1.9 |
0.8 |
3.2 |
||
|
ADHD |
45 |
2.7 |
1.8 |
10.0 |
0.661a |
|
|
Non-ADHD |
66 |
3.3 |
4.0 |
28.0 |
||
|
BMI-for-age- Z score |
|
|||||
|
BAZ1 |
< -3SD |
3 |
2.1 |
0.099b |
||
|
BAZ2 |
< -2SD |
1 |
1.9 |
|||
|
BAZ3 |
-2 to 1 SD |
53 |
3.3 |
4.2 |
28.0 |
|
|
BAZ4 |
> 1SD |
12 |
2.7 |
2.5 |
10.0 |
|
|
BAZ5 |
> 2SD |
22 |
2.8 |
1.9 |
10.0 |
|
|
No BAZ data |
|
20 |
3.0 |
2.6 |
12.0 |
|
Table 1: The hair iodine levels in μg/gm among the different age, sex, address, school types, religious, ADHD, and BAZ-nutritional status categories.
Note: * P-value < 0.05, a Mann-Whitney U test, b Kruskal Wallis Test
ADHD: Attention Deficit and Hyperactive Disorders; BAZ: BMI-for-age-Z score; BAZ1: Severely wasted (< 5-year) or Severely thinness (>5-year); BAZ2: Wasted (< 5-year) or Thinness (>5-year); BAZ3: Normal; BAZ4: Possible overweight (< 5-year) or overweight (>5-year); BAZ5: Overweight or Obese (< 5-year) or Obese (>5-year).
|
Quartiles |
1st |
2nd |
3rd |
4th |
|
|
Hair iodine levels(ug/gm) |
<1.5 |
1.5-2.3 |
2.3-3.0 |
>3.0 |
P-value |
|
< 5-year-old |
12 |
10 |
10 |
13 |
0.667 a |
|
> 5-year-old |
16 |
20 |
12 |
18 |
|
|
Girls |
5 |
11 |
9 |
15 |
0.028 a, * |
|
Boys |
23 |
19 |
13 |
16 |
|
|
Lived in Bangkok |
15 |
24 |
17 |
27 |
0.045 a, * |
|
Lived in the Perimeters |
13 |
6 |
5 |
4 |
|
|
Private School |
17 |
22 |
20 |
26 |
0.005 a, * |
|
Public School |
11 |
8 |
2 |
5 |
|
|
Buddhist |
24 |
25 |
20 |
30 |
0.117 a |
|
Non-Buddhist |
4 |
5 |
2 |
1 |
|
|
ADHD |
10 |
10 |
11 |
14 |
0.312 a |
|
Non-ADHD |
18 |
20 |
11 |
17 |
|
|
BAZ1 |
0 |
1 |
0 |
0 |
0.045 a, * |
|
BAZ2 |
0 |
1 |
0 |
0 |
|
|
BAZ3 |
15 |
11 |
12 |
14 |
|
|
BAZ4 |
3 |
4 |
3 |
2 |
|
|
BAZ5 |
3 |
8 |
5 |
6 |
|
|
no BAZ data |
7 |
6 |
2 |
8 |
|
|
Total |
28 |
31 |
22 |
30 |
0.343 a |
Table 2: The frequency of participants among the four quartiles of hair iodine levels.
Note: * P-value < 0.05, a Chi-Square Test. 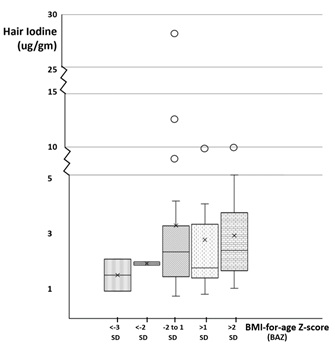
Figure 1: A box and plot graph of the hair iodine levels distributions among the different BAZ-nutritional status categories.
Discussion
Humans excrete most dietary iodine intakes through urine within a few days; hence the median UIC level is recognized as the iodine exposure indicator for the epidemiological studies [2]. However, the urine iodine level reflexes a relatively short-term exposure of only a few days of intake. The UIC levels could also vary daily, or even within the same day, in an individual [1,2,22,23]. These variations make the UIC level not very useful as the exposure indicator for an individual exposure assessment [24]. Despite the unsettling debates on inter- and intra-laboratory variations of the hair-element analytical processes [19], previous studies suggested hair iodine level as a valuable long-term-exposure indicator [25-27].
There was no previous reference for the hair iodine level in Thai children. We can not derive the practical cut-off level of hair iodine from this study dataset with ROC analysis. Previous studies reported the median and average ICP-MS-derived hair iodine levels between 0.385-0.826 and 1.12-1.13μg/gm, respectively, in the healthy subjects, as summarized in table 3. Our analysis reported the median of hair iodine levels, at 2.3μg/gm, and average±S.D, at 3.1±3.3, were considerably higher than those of the previous findings. The high outliers and a broad S.D range in the Thai children data set also indicated the exceptional high exposure in the vulnerable groups.
|
References |
N |
Groups |
Age (Years) |
Hair Iodine Level (μg/gm) |
Methods |
|
Skalny et al. [28] |
74 |
Sex and age-matched Control group |
2-4 5-9 |
Median 0.63 Range 0.349-1.160 (all) Median 0.826 Range 0.491-1.283 (2-4 years) Median 0.443 Range 0.195-0.917 (5-9 years) |
ICP-MS |
|
Skalny et al. [28] |
33 |
Control group |
3-8 |
Median 0.719 Range 0.523-1.381 (3-4 years) Median 0.477 Range 0.190-0.800 (>4years) |
ICP-MS |
|
Momcilovic et al. [27] |
870 |
270 males 600 females |
adults |
Median 0.50 Range 0.022 -15.45 Optimum 0.57-0.74 |
ICP-MS |
|
Blaurock-Busch et al. [29] |
146 |
Non-autistic children |
3-9 |
7.9 (95th percentile) |
ICP-MS |
|
Prejac et al. [26] |
246 |
90 males 156 females |
adults |
Median 0.501 (all) (0.511M, 0.500 F) |
ICP-MS |
|
Ochi et al. [30] |
100 |
Males |
adults |
Median 0.385 Optimal 0.209-0.535 (25%-75% Percentiles) |
ICP-MS |
|
Adams et al. [31] |
40 |
Neurotypical Children 30 males 10 females |
7.5 ± 3.0 |
Mean 1.13 (3-15 years) Mean 1.12 (3-6 years) SD 1.02 |
ICP-MS |
|
Druyan et al. [25] |
|
Healthy American Population Study |
1-11 |
Median 0.78 Optimal 0.250-1.300 1.30 (95th percentile) |
ICP-MS |
Table 3: The hair iodine levels in the healthy subjects from the previous ICP-MS studies.
Note: ICP-MS: Induction coupled plasma mass spectrometry.
There were several reports of the excessive iodine intake status in the school-age children in Korea, Nepal, China, East Africa, Algeria, and Colombia [4,16,31-37]. Such findings raise the concern of excessive iodine intake in a specific group of Thai children.
A serial of 2000, 2012, and 2014 national surveysreported the median UIC levels in the school-age Thai children at 150, 262, and 168μg/gm, respectively [4,21,38]. There were also the broad ranges of UIC levels from 20 to 2,495ug/L. Altogether, 42% of children had their median UIC levels above 200μg/L, which indicated either the above-the-requirement or excessive iodine exposure by the WHO's iodine intake criteria [2,38]. Moreover, 19% of children had their UIC levels below 100μg/L, which was the cut-off level for insufficient iodine intake status [2].
High outliers in urine and hair iodine studies indicated the risk of excessive iodine exposure in the susceptible children group. Unlike the known association of iodine insufficiency in the malnourished children, our study showed the excess hair iodine status in the group of well-nourished children.
There were no differences between the age groups in this hair iodine study. But the Thai national survey showed higher median UIC levels in young children than those in the school-aged group [38]. Bangkokian and private-school students also prone to excessive hair iodine status.
In this analysis, the hair iodine levels were evenly distributed from the first to the fourth quartile groups, which suggested the co-existing of insufficient, adequate, and excessive iodine intake status among these children. The double edges of the iodine intake status, ranging from inadequate to the potentially excessive intake, were evident in these children. Therefore, the young and well-nourished Bangkok children from middle to high socioeconomic status families were likely the high-risk group for excessive iodine exposure.
In 1995, 2001, 2009, and 2014 national surveys, the proportions of Thai overweight and obese children were gradually increasing [38,39]. The 2014 survey reported the percentages of overweight and obese children in Bangkok at 46 and 51% in the 1-5 and 6-11-year-old groups, respectively [38]. Such trends suggested an imminent risk of excessive iodine intake in these well-, or overnourished children.
The recent national survey did not find the association of the iodine levels in the household table salts to the children's UIC levels [38]. These findings warranted the proper risk assessment studies to identify potential iodine exposure sources, the exposure levels, the potential health impacts, and the future risk management strategies.
This analysis's main limitation was the lack of dietary intake information to help relating dietary iodine sources to these hair iodine findings. Another disadvantage was the analysis of a hair iodine dataset, which was not the standard iodine exposure marker. However, together with the nature of long-term iodine retention in hair samples and the precise handling of hair sample collection, handling, processing, and analyzing, several studies supported the hair iodine level's validity as an individual long-term exposure indicator. These overall findings suggested the potential risk of excessive iodine intake in the specific young, well-nourished children from the middle to high-income families of Bangkok.
Declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of Mahidol Universitywith a certificate of approval (COA) No.MU-CIRB 2015/125.2010 and registered in Thai Clinical Trials Registry (TCTR) with study ID of 20151113001. All participants provided written consent.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request
Competing interests
All authors declare no conflict of interest.
Funding
The National Research Council of Thailand funded the original study.
Author’s contribution
T.T. and AY analyzed and interpreted the study dataset and wrote the manuscript. D.T. reviewed and provided suggestions and comments. All authors read and approved the final manuscript.
Acknowledgment
We are grateful for the Healing Passion Medical Center staff for their supports during the data gathering and analytical processes.
References
- Rohner F, Zimmermann M, Jooste P, Pandav C, Caldwell K, et al. (2014) Biomarkers of nutrition for development-iodine review. J Nutr 144: 1322-1342.
- WHO (2013) Urinary iodine concentrations for determining iodine status in populations. In: Vitamin and Mineral Nutrition Information System. World Health Organization, Geneva, Switzerland.
- WHO/NHD/01.1 (2007) Assessment of iodine deficiency disorders and monitor-ing their elimination: A guide for programme managers. World Health Organization/UNICEF/International Council for the Control of Iodine Deficiency Disorders, Geneva, Switzerland.
- IGN (2019) Global scorecard of iodine nutrition in 2019 in the general population based on school-age children (sac). The Iodine Global Network, Zurich, Switzerland.
- Leung AM, Braverman LE (2014) Consequences of excess iodine. Nature reviews. Nat Rev Endocrinol 10: 136-142.
- Galofre JC, Fernandez-Calvet L, Rios M, Garcia-Mayor RV (1994) Increased incidence of thyrotoxicosis after iodine supplementation in an iodine sufficient area. J Endocrinol Invest 17: 23-27.
- Todd CH, Allain T, Gomo ZA, Hasler JA, Ndiweni M, et al. (1995) Increase in thyrotoxicosis associated with iodine supplements in zimbabwe. Lancet 346: 1563-1564.
- Kahaly GJ, Dienes HP, Beyer J, Hommel G (1998) Iodide induces thyroid autoimmunity in patients with endemic goitre: A randomised, double-blind, placebo-controlled trial. Eur J Endocrinol 139: 290-297.
- Parveen S, Latif SA, Kamal MM, Uddin MM (2007) Effects of long term iodized table salt consumption on serum t3, t4 and tsh in an iodine deficient area of Bangladesh. Mymensingh Med J 16: 57-60.
- Thomson CD, Campbell JM, Miller J, Skeaff SA (2011) Minimal impact of excess iodate intake on thyroid hormones and selenium status in older new zealanders. Eur J Endocrinol 165: 745-752.
- Blomberg M, Feldt-Rasmussen U, Andersen KK, Kjaer SK (2012) Thyroid cancer in denmark 1943-2008, before and after iodine supplementation. Int J Cancer 131: 2360-2366.
- Sang Z, Wang PP, Yao Z, Shen J, Halfyard B, et al. (2012) Exploration of the safe upper level of iodine intake in euthyroid chinese adults: A randomized double-blind trial. Am J clin Nutr 95: 367-373.
- Dong W, Zhang H, Zhang P, Li X, He L, et al. (2013) The changing incidence of thyroid carcinoma in shenyang, china before and after universal salt iodization. Med Sci Monit 19: 49-53.
- Leung AM, Avram AM, Brenner AV, Duntas LH, Ehrenkranz J, et al. (2015) Potential risks of excess iodine ingestion and exposure: Statement by the american thyroid association public health committee. Thyroid 25: 145-146.
- Andersen SL, Laurberg P (2016) Iodine supplementation in pregnancy and the dilemma of ambiguous recommendations. Eur Thyroid J 5: 35-43.
- Katagiri R, Yuan X, Kobayashi S, Sasaki S (2017) Effect of excess iodine intake on thyroid diseases in different populations: A systematic review and meta-analyses including observational studies. PloS One 12: 0173722.
- Tippairote T, Temviriyanukul P, Benjapong W, Trachootham D (2016) A pilot case-control study of lead and other hazardous elements in hair samples and risk of attention deficit and hyperactivity disorders in thai children. Thai J Toxicology (NCT7): 75-83.
- Tippairote T, Temviriyanukul P, Benjapong W, Trachootham D (2017) Hair zinc and severity of symptoms are increased in children with attention deficit and hyperactivity disorder: A hair multi-element profile study. Biological Trace Element Research 179: 185-194.
- Wolowiec P, Michalak I, Chojnacka K, Mikulewicz M (2013) Hair analysis in health assessment. Clin Chim Acta 419: 139-171.
- WHO (2006) Who child growth standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. World Health Organization, Geneva, Switzerland.
- de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, et al. (2007) Development of a who growth reference for school-aged children and adolescents. Bull World Health Organ 85: 660-667.
- Rasmussen LB, Ovesen L, Christiansen E (1999) Day-to-day and within-day variation in urinary iodine excretion. Eur J Clin Nutr 53: 401-407.
- WHO (2007) Assessment of iodine deficiency disorders and monitoring of their eliminination: A guide for programme managers, 3rd ed. World Health Organization, Geneva, Switzerland.
- Andersson M, de Benoist B, Delange F, Zupan J (2007) Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: Conclusions and recommendations of the technical consultation. Public Health Nutr 10: 1606-1611.
- Druyan ME, Bass D, Puchyr R, Urek K, Quig D, et al. (1998) Determination of reference ranges for elements in human scalp hair. Biological Trace Element Research 62: 183-197.
- Prejac J, Mimica N, Barbot I (2012) Incommensurability of human hair and whole blood iodine. Trace Elements in Medicine 13: 20-24.
- Momcilovic B, Prejac J, Višnjevic V, Skalnaya MG, Mimica N, et al. (2013) Hair iodine for human iodine status assessment. Thyroid 24: 1018-1026.
- Skalny AV, Simashkova NV, Klyushnik TP, Grabeklis AR, Bjørklund G, et al. (2017) Hair toxic and essential trace elements in children with autism spectrum disorder. Metab Brain Dis 32: 195-202.
- Blaurock-Busch E, Amin OR, Dessoki HH, Rabah T (2012) Toxic metals and essential elements in hair and severity of symptoms among children with autism. Maedica (Bucur) 7: 38-48.
- Ochi T, Fujiwara H, Okamoto S, An J, Nagai K, et al. (2011) Novel adoptive T-cell immunotherapy using a WT1-specific TCR vector encoding silencers for endogenous TCRs shows marked antileukemia reactivity and safety. Blood 118: 1495-1503.
- Adams R, Bessant J, Phelps R (2006) Innovation management measurement: A review. International Journal of Management Reviews 8: 21-47.
- Zimmermann MB (2013) Iodine deficiency and excess in children: Worldwide status in 2013. Endocr Pract 19: 839-846.
- Du Y, Gao Y, Meng F, Liu S, Fan Z, et al. (2014) Iodine deficiency and excess coexist in china and induce thyroid dysfunction and disease: A cross-sectional study. PloS One 9: 111937.
- Aakre I, Strand TA, Bjøro T, Norheim I, Barikmo I, et al. (2016) Thyroid function among breastfed children with chronically excessive iodine intakes. Nutrients 8: 398.
- Choi YS, Ock S, Kwon S, Jung SB, Seok KH, et al. (2017) Excessive iodine status among school-age children in korea: A first report. Endocrinol Metab (Seoul) 32: 370-374.
- Jessica F, Michael BZ, Fatma A, Vincent A, Ralph F, et al. (2018) Effect of excess iodine intake from iodized salt and/or groundwater iodine on thyroid function in nonpregnant and pregnant women, infants, and children: A multicenter study in East Africa. Thyroid 28: 1198-1210.
- Tamang MK, Gelal B, Tamang B, Lamsal M, Brodie D, et al. (2019) Excess urinary iodine concentration and thyroid dysfunction among school age children of eastern nepal: A matter of concern. BMC Res Notes 12: 294.
- Wichai A, Ladda M, Nichara R, Warapone S, Hathichanok P (2018) National health examination survey. Health Information System Development Office.
- Wichai A (2011) National health examination survey. Health Information System Development Office.
Citation: Tippairote T, Yaovapak A, Trachootham D (2022) High Iodine Status in the Hair Samples of Well-Nourished Thai Children. J Food Sci Nutr 8: 136.
Copyright: © 2022 Torsak Tippairote, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

