
Hypolipidemic Effect of Supplements Containing the Bioactive Compounds Found in Amazonian Fruits
*Corresponding Author(s):
Adele Salomão-OliveiraMulti-Institutional Post-Graduate Biotechnology Program, Federal University Of Amazonas (FUA), Manaus, Brazil
Tel:+55 92982217372,
Email:adelesalomao@gmail.com
Abstract
Objective
To ascertain the hypolipidemic effect of the bioactive compounds found in Amazonian fruits by feeding dyslipidemia-induced rats, on a formulation prepared with these bioactive compounds.
Methods
Murine, biochemical and histological evaluations were performed and observed by light microscopy during the 37 days of the experiment. Pre-clinical trials were performed on 30 rats (Wistar strain (Rattus norvegicus), which were divided into five randomly distributed groups (G) (n=6).
Results
The formulation showed free-radical scavenging activity in ABTS· +, DPPH· and FRAP. It also exhibited cellular antioxidant activity in vitro in a concentration-dependent manner, when compared to G1 and G3. It significantly decreased (p<0.01) the levels of total cholesterol, triglycerides, LDL, VLDL, OGT and PGT (p<0.05) and significantly increased (p<0.05) those of HDL. In addition, the formulation did not cause chronic renal, hepatic and neurotoxic toxicity, confirming its exceptionally beneficial effect on adipose and hepatic tissues in the groups of rats treated.
Conclusion
Our data demonstrated that the use of this supplement containing the bioactive compounds found in the Amazonian fruits produced a lipid-lowering effect, and contributed to minimizing the complications brought about by the excess of serum fats.
Keywords
Amazonian fruits; Animal model; Antioxidants; Bioactive compounds; Dyslipidemias
Introduction
Chronic diseases, mainly those caused by inadequate lifestyle, were responsible for 71% of the 41 million deaths in 2018 [1] and it is expected that, by 2030, they will be the cause of 72% of all deaths in Brazil [2]. Obesity is considered an epidemic, with an estimated 2,025 out of 300 million obese people worldwide According to a survey carried out in 2018 by the World Health Organization (WHO) [1]. In Brazil, it affects approximately 21% of the adult population and is mainly related to dyslipidemia, and is becoming a major challenge when it comes to its prevention and treatment within the Brazilian public health system [2].
Dyslipidemia alters the lipid profile and causes an increase in the levels of CT, TG, LDL-c and a decrease in those of HDL-c, as a consequence, dyslipidemia is considered to be a major determinant of the severe metabolic problems [3].
The chemical and nutritional characteristics of the Amazonian fruits camu-camu (Myrciaria dubia (Kunth) McVaugh), açaí (E. precatoria Mart.) and guaraná (Paullinia cupana) arouse interest due to their high content of bioactive compounds and antioxidants, in addition to possessing micronutrients, which are essential for the proper functioning of the organism, such as ascorbic acid, phenolic compounds and methylxanthines. These have protective effects on the development and progression of dyslipidemia and atherosclerosis, as well as an important impact on cardiovascular risk and other noncommunicable chronic diseases [4,5].
Because of their therapeutic value, bioactive compounds from plants and fruits are used frequently and, in many developing countries, about 80% of the medicines available come from medicinal plants but, in industrialized countries, they constitute the raw material for processes, which synthesize pure chemical derivatives, thus minimizing the side effects caused by allopathy [6].
The present study shows the use of Ascorbic Acid (AA), which is prevalent in camu-camu, and involved in important metabolic and endocrine processes involved in the genesis and control of weight, as well as being a protective factor for dyslipidemia [7]. When camu-camu is associated with the açaí and guarana, together they become an exceptional source of bioactive compounds [8]. Thus, these native Amazon fruits possess interesting bioactive compounds and sources of natural antioxidants, and indicate promising perspectives for the development of formulations derived from bioproducts. These formulations could be of great impact in the reduction of the deleterious effects of dyslipidemia, oxidative stress, and their resultant implications for NCDs and comorbidities.
Methods
Elaboration of the supplement
Raw extracts of the camu-camu fruits (Myrciaria dubia (kunth) Mc Vaugh), açaí (Euterpe precatoria Mart.) and guaraná (Paullinia cupana) were prepared in the Chemistry of Natural Products Laboratory / UFAM and the Chemistry of Natural Products laboratory at the Amazonian Biotechnology Center, based on a methodology adapted from Rufino et al. [9].
The patent for the formulation containing Amazonian fruits is under the protection of the National Institute of Industrial Property (INPI) under the Process number: BR 10 2018 068302 0 (unpublished data).
Physico-chemical analyses of the supplement
Hydrogen potential (pH) was analyzed using a digital pH meter, Quimistm, mod: Q400AS, 90-240V, 10W, following the AOAC method [10]. While the water activity (Wa) was analyzed using an activity meter (AquaLab-Dew Point Water Activity Meter 4 TE, mod. S4TE, 110w BrasEq- Brasileira de Equipamento Ltda) at 25ºC using the method described by Piga et al. [11].
The process used for extraction of ascorbic acid were performed by HPLC according to Campos et al. [12]. Ascorbic acid was determined using HPLC (Shimadzu) equipped with Shim-pack CLC-ODS (M) ®C18 Shimadzu 250×4.6mm, 5μm chromatographic column. The flow of the mobile phase (1mM NaH2PO4, 1mM EDTA, pH 3.00) and its running time were 1.0mL.min-1 and 3.0min, respectively. Elution was detected using UV-VIS detector (Shimadzu) with wavelength set at 245 nm. For the comparison of the absorption spectra of the formulation, the Merck standard acid L (+) - ascorbic was used.
The analyses of the total phenolic compounds present in the crude sample extracts were analyzed using the Folin-Ciocalteu method [13]. The standard curve of gallic acid, according to the method proposed by Singleton et al. [14] and modified by Rufino et al. [9], was prepared under light protection, and the absorbance was measured in a UV / VIS-552a (Perkin-Elmer) spectrophotometer at 740nm.
The determination of anthocyanin content was performed by the single pH method, according to the method described by Fuleki and Francis [15]. The 1mg aliquot of the formulation was diluted in ethanol: 1.5 N HCl (85:15) v/v and the UV-VIS (1601 Pc Mark Shimadzu) spectrophotometer was read at a 535nm length and obtained an absorbance (Abs) of approximately 0.393. Absorbance values were contrasted with white values (Ethanol Solution: 1.5 N HCl (85:15)) and the value of 982 was adopted for the single pH method (pH=2.0).
Characterization of bioactive compounds by UHPLC-DAD-MS/MS
The nutraceutical sample was diluted to 1mg mL-1 in methanol: ultrapure water (50:50) v/v and analyzed (10μL, injection volume) with an ultra-high pressure liquid chromatography tandem mass spectrometry (UHPLC-DAD-MS/MS) system, consisting of an Accela UHPLC (Thermo Scientific, CA, USA) coupled to a triple quadrupole mass spectrometer model TSQ Quantum Access (Thermo Scientific, MA, USA) equipped with an Atmospheric Pressure Chemical Ionization (APCI) interface running in both positive and negative ion mode. Gradient elution was performed according to a previously modified method [16]. UV spectra were registered from 240nm to 400nm and the tentative identification of the bioactive compounds was performed by comparing MS/MS spectra with previously published data.
DPPH•
The DPPH sequestration method (IC50) was performed according to methodology used by Sousa et al. [17]. Samples and standard stock solutions were prepared at the concentration 1mg/mL using Tedia brand methanol as a solvent. The microplate reading was performed on the ultraviolet spectrometer at 517nm.
ABTS+
ABTS analysis was evaluated according to the method by Re et al. [18], using Trolox equivalence, and a concentration of 1000μg/mL. Absorbance of the solution was measured at 734nm using a UV-vis spectrophotometer (Thermo Scientific, Evolution 220). Trolox was used in the calibration curve (100 to 2000μM). The results were expressed in μM of Trolox Equivalent Antioxidant Capacity (TEAC) per gram of the formulation.
FRAP
For the FRAP test, the concentration of 1000 and 500μg/mL was used, in which the concentration of 1000μg entered the curve, assuming an activity expressed by equivalence to the standard Ferrous Sulfate [19]. A sample aliquot (90μL, 7.8μg/mL) was mixed with 270μL ultrapure water and added to 270μL FRAP reagent. After incubation for 30min at 37°C, the absorbance was measured at 595nm using a UV-vis spectrophotometer (Thermo Scientific, Evolution 220).
Determination of caffeine
The determination was performed by HPLC following the methodology proposed by Camargo and Toledo [20].
Evaluation of enzymatic and cellular activities in vitro
The supplement was diluted to 1mg/mL and then added to 96-well microplates with the enzymatic solutions (pancreatic lipase, Aspergillus oryzae α-amylase and rat intestinal α-glycosidase). For the preparation of the α-glycosidase enzyme solution, the samples were centrifuged at 3500rpm for 10min and the microplate readings were performed on the spectrometer in the ELISA reader at 405nm absorbance according to the methods used by Slanc et al. [21], Subramaniam et al. [22] and Andrade-Cetto et al. [23].
Cellular Antioxidant Activity (CAA)
The evaluation of cellular antioxidant activity was performed using the methodology described by Wolfe and Liu [24]. The CAA assay was performed to quantify the antioxidant activity of the supplement based on the detection of intracellular ROS production using the fluorescent compound: 2'7'-Dichloro-Fluorescein-Diacetate (DCFH-DA). In this technique, cells of the MRC-5 lineage were obtained from the Rio de Janeiro Cell Bank (RJCB).
Experimental study
A total of thirty 22 day-old male and female rats of the Wistar strain (Rattus norvegicus) (57±11g body weight), were obtained from the Animal Laboratory at INPA (Amazonian National Research Institute, AM, Brazil). The rats were subjected to 12h light/dark cycle at a temperature of 22±2°C and a relative humidity of 40±10% for 37 days. During the acclimation period, the animals were housed in polycarbonate cages and fed on a basal diet and hypercaloric, hyperlipidic diet with water ad libitum. The water containers were replenished weekly while their food containers were weighed and filled daily and the feed leftovers were weighed and measured before each change to assess the animals’ intake following the methodology used by Nascimento et al. [25].
After acclimation, the rats were randomly divided into 5 groups with 6 rats in each group, their weights were monitored weekly using a semi-analytical balance and, after 37 days of obesity induction via hypercaloric and hyperlipidic diet, the treatments were started with simvastatin and given a supplement based on Amazonian fruits at the doses of 20mg/0.5mL and 40mg/0.5mL by oral gavage. Additionally, a control group was given an equivalent volume of the vehicle (normal saline solution) and basal feeding (commercial food). The physiological properties of the rats were observed throughout the experimental period (37 days), and the physical signs were observed daily according to an adopted protocol. This analyzed the behavior of the fur, peripheral effects, quantification of water and feed consumption, lethality and reversibility of symptoms stimulation and central depression, recording the present, absent or gradual intensity [26].
We also noted some clinical symptoms of chronic toxicity, such as, lack of motor coordination, and/or corneal reflex, tail pinch response, gait (ataxia), muscle tone, irritability, touch response, nervous system related changes (tremors, seizures, sedation, analgesia, lacrimation, palpebral ptosis, defecation, polyuria, piloerection and respiratory rhythm), changes in body weight, according to the methodology proposed by Malone and Robichaud [27].
All experimental methods were approved by the Ethical Committee on the Use of Animals at the Amazonian National Research Institute, and certified under review no 007/2017, according to the norms that establish the procedures for the scientific use of animals in Brazil.
Animal grouping
The 5 groups were treated according to the following: Group A was control and received commercial feed, Group B was subjected to induced obesity with consumption of the hypercaloric and hyperlipid diet is under the patent protection from the National Institute of Industrial Property (INPI) under the Process number: BR 10 2020 002474 4 (unpublished data), Group C was subjected to induced obesity with the consumption of the hypercaloric and hyperlipid diet ad libitum and treated with simvastatin (50mg/kg/day) according to the studies by Liu et al. [28], by gavage once a day for 37 days, Group D was subjected to induced obesity with the hypercaloric and hyperlipid diet and given 100mg/kg/day of the formulation derived from the Amazonian fruits and Group E was subjected to induced obesity with the hypercaloric and hyperlipid diet and given 200mg/kg/day of the formulation derived from the Amazonian fruits in 0.5mL solution by gavage once a day for 37 days.
During the study period, 3 blood samples were collected from each rat’s caudal vein following an overnight fasting period. The first sampling was performed when the rats were 22 days-old (T1 T=time), the second one after the induction of obesity (at 15 days), e.g. T2, and the third one (T3) in the period of 22 days after the induction of obesity (T2), in order to analyze the effect of the treatments on supplement and simvastatin concentrations on biochemical markers and ended on the day the animals were euthanized (T3).
At the end of the test, all animals were fasted for 8 hours. Then they were anesthetized with Ketamine 0.5mL/kg and 0.1mL/kg intraperitoneal (PI) Xilasin and euthanized. Blood from the cardiac puncture was collected and 5mL blood samples were stored in tubes containing sodium heparin (125 UL-1) and immediately kept on ice. After this procedure, samples were centrifuged at 3000rpm for 5minutes at 4°C and the plasma obtained was stored in a freezer at -80°C until dosages of biochemical determinations were performed. All analyses were determined in a Cobas Mira ® automatic analyzer (Roche Diagnostics), using BIOCLIN ® kits in the Biological Activity and Cell Culture Laboratory- BioPHAR (FCF/FUA).
Haematological and histological studies
The serum TC, TG, LDL-c, HDL-c, blood glucose, GOT and GPT were measured using commercial kits BIOCLIN ® in a Cobas Mira® automatic analyzer (Roche Diagnostics) according to the manufacturer’s protocols.
The liver and unilocular and multilocular adipose tissue were removed and weighed immediately after euthanasia and stored in 10% buffered formalin according to Bancroft and Stevens [29], in the Functional Histology Laboratory (FUA).
Statistical analysis
Values are presented as the mean and SD. The results of five groups were analyzed by Prism 6.0 software using one-way ANOVA with Tukey’s test, considering differences between controls and exposed animals to be statistically significant when p<0.05.
Results
Physico-chemical characterization of supplement
Table 1 shows the results of the tests related to the pH, Wa and the antioxidant activities determined by the DPPH, ABTS and FRAP methods, as well as the content of antioxidant compounds such as ascorbic acid and phenolic compounds. Thus, the levels verified in the formulation demonstrate it to be an excellent source of antioxidant compounds.
|
Determinations |
Supplement |
|
pH |
3.22+0.01 |
|
Wa |
0.39±0.03 |
|
Ascorbic acid (g.100-¹) |
19,845.00±7.77 |
|
Caffeine (g) |
8.00±0.23 |
|
Total phenols (mg EGA.100g-1) |
12,206.00±0.41 |
|
Anthocyanins |
6,558.00±1.23 |
|
DPPH (ic50μg/mL) |
24.30±0.36 |
|
ABTS (μmol/g) |
1,245.66±1.93 |
|
FRAP |
1,115.25±4.51 |
Table 1: Chemical analysis and bioactive compound content of the Amazonian fruit based supplement.
Note: GAE: gallic acid equivalent. Results are expressed as mean ± standard deviation.
Characterization of bioactive compounds by UHPLC-DAD-MS/MS
The HPLC-DAD-MS/MS analysis displayed four main bioactive compounds in the nutraceutical sample. The peaks 1 (Rt 3.06min), 2 (Rt 5.36min), 3 (Rt 6.40min), and 4 (Rt 6.88min) presented protonated base peaks at m/z177, 291, 195, and 291, respectively. Based on manual interpretation of MS/MS spectra and comparison with previously published data [30-32], these bioactive compounds were tentatively identified as ascorbic acid, catechin, caffeine, and epicatechin (Figure 1).
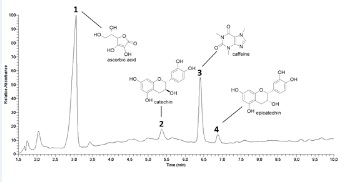 Figure 1: Supplement sample chromatogram at 240-400nm.
Figure 1: Supplement sample chromatogram at 240-400nm.
In vitro enzymatic tests
The present study ascertained the effect of the formulation on some enzymes such as α-amylase, α-glycosidase, and lipase. The tests performed showed there to be no inhibition of the digestive enzymes, and observed an activity index of less than 50%.
Cell antioxidant activity
The CAA assay shows the formulation’s antioxidant activity to have started at the concentration of 25μg/mL, as shown in figure 2.
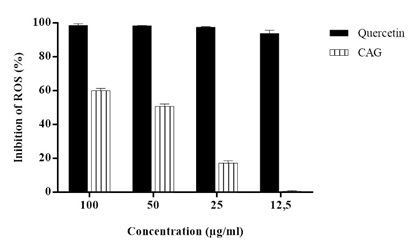 Figure 2: Percent inhibition of Reactive Oxygen Species (ROS) of standard Quercetin and supplement.
Figure 2: Percent inhibition of Reactive Oxygen Species (ROS) of standard Quercetin and supplement.
Note: Data were analyzed by one-way ANOVA. There was a significant difference between the means of the supplement and the standard Quercetin (p <0.01). There were differences between the averages of supplement concentrations (p <0.01) and in relation to the standard Quercetin there was significant difference (p> 0.05) between them.
Animal studies
Experimental design
Murinometric parameters: The mean body weights showed no significant difference between treatments, yet the group that consumed 200mg/kg/day of the supplement had the largest weight variation. The same was observed regarding the control and experimental groups murinometric parameters (Lee index - LI and waist circumference -WC) data, and showed no statistical difference (p>0.05) between treatments, however, the group that received 200mg/kg/day of the supplement presented the lowest value (LI and WC) in the assessments.
In the variance analysis of weekly consumption versus supplement treatment during the study, it was also observed that the experimental groups of animals did not differ according to the one-way ANOVA test followed by the F test (p≥0.05).
Haematological parameters and histological examination
Figures 3 and 4 show the results of the biochemical parameters. These indicate that the supplement demonstrated a significant improvement (p<0.05) in the lipid profile, as well as a significant decrease (p<0.05) in hepatic transaminase (PGT and OGT), when comparing groups of control animals to the experimental group that used simvastatin. However, there was no improvement in glucose in any experimental group, and no significant decrease (p≥0.05) of this parameter was observed in relation to all treatments.
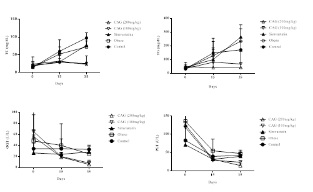 Figure 3: Treatment days and TC, TG, PGT and OGT values from analysis of variance.
Figure 3: Treatment days and TC, TG, PGT and OGT values from analysis of variance.
Note: Comparisons for the means of TC, TG, VLDL, TGP and OGT by means of the F and Tukey test. The supplement of CAG had significant differences (p<0.05) in the decrease of TC, TG, VLDL-c, TGP and OGT compared to the other treatments (obese and simvastatin).
 Figure 4: Treatment times and the LDL-c (A) and HDL-c (B) values analysis of variance.
Figure 4: Treatment times and the LDL-c (A) and HDL-c (B) values analysis of variance.
Note: Comparisons for the means of LDL-c and HDL-c by means of the F and Tukey tests. The supplement of CAG had significant differences (p<0.05) in the decrease of LDL-c and in the increase of HDL-c compared to the other treatments (obese and simvastatin).
Adipose and liver tissue in rats
The Multilocular (MAT) and Unilocular Adipose Tissue (UAT) of the visceral tissue and liver tissue, respectively, of the animals in all groups were analyzed with standard histochemistry (Figure 5). However, those of the groups in which obesity had been induced and had consumed simvastatin (groups B and C) presented hyperplasia of the multilocular adipocytes and inflammatory infiltrate (C). There was a small reduction in these changes in the group that consumed 100mg/kg of the supplement (D), when compared to the control group (A). While in the group that consumed 200mg/kg of the supplement (E) hypoplasia and hypotrophy showed to have obtained an improvement in dyslipidemia. However, the group that received the highest concentration of the supplement (E) was the one that presented the best result when compared with the other experimental groups (B-D), and in which was observed the increase in the MAT, a phenomenon called transdifferentiation [33].
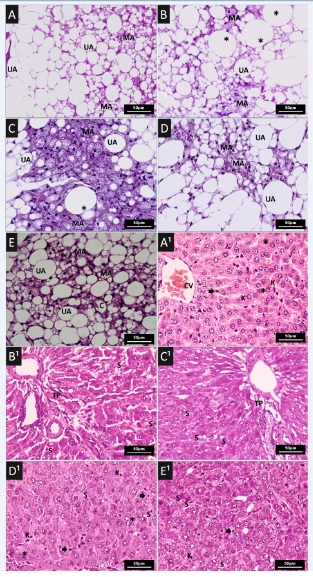 Figure 5: Histopathological features in different animal groups. (A-E) Representative Haematoxylin and Eosin (H&E) staining of adipose tissue sections of: (A) Wistar rat fed a control diet; (B) induced obesity with consumption of experimental ration (hypercaloric and hyperlipid; (C) induced obesity with consumption of experimental ration and treated with simvastatin (50mg/kg/day) by gavage once a day for 35 days; (D and E) induced obesity with consumption of experimental ration and treated with supplement with 100mg/kg/day and 200mg/kg/day, respectively, by gavage once a day for 35 days. Unilocular Adipocyte (UA), Multilocular Adipocyte (MA), Hypertrofic adipocyte (*), Capillary vessel (c).
Figure 5: Histopathological features in different animal groups. (A-E) Representative Haematoxylin and Eosin (H&E) staining of adipose tissue sections of: (A) Wistar rat fed a control diet; (B) induced obesity with consumption of experimental ration (hypercaloric and hyperlipid; (C) induced obesity with consumption of experimental ration and treated with simvastatin (50mg/kg/day) by gavage once a day for 35 days; (D and E) induced obesity with consumption of experimental ration and treated with supplement with 100mg/kg/day and 200mg/kg/day, respectively, by gavage once a day for 35 days. Unilocular Adipocyte (UA), Multilocular Adipocyte (MA), Hypertrofic adipocyte (*), Capillary vessel (c).
Note: Binucleated hepatocytes (*), Kuppfer cells (K), Steatosis (S), Portal Triad (PT), Centrolobular Vein (CV).
The effects of treatments on hepatic tissue are shown in figure 5. In the liver section of the obesity-induced groups, which had consumed simvastatin, the structure of hepatic lobular tissue was clear and intact. However, we did not the presence of mixed steatosis and inflammatory infiltrate (B1 and C1), when compared with the control group (A1). In relation to the groups of rats that received treatment with the supplement (D1 and E1), improvement of mixed hepatic steatosis and inflammatory infiltrate was observed (D1 and E1).
Discussion
The formulation was classified as very acidic (3.22+0.01), as shown in Table 1, considering the following classification: (Low Acid: pH>4.5), (Acid: pH 4 to 4.5) and (Very Acidic pH<4.0) [34]. This "very acidic" characteristic be related to a high concentration of ascorbic acid and is a very important and intrinsic factor in limiting the growth of microorganisms, enzymatic activities, retention of flavor and the general conservation of the bioactive compounds of the supplement.
It was also observed that the nutraceutical formulation offers microbiological safety due to its Water activity (Wa). The determination of water activity is a very important factor and is being adopted in several countries as a compulsory standard for food preservation, when the Wa scale is between (0.60 and 1.0) [35]. The lowest Wa limit in which a microorganism can grow in food is around 0.60. The results of pH and Wa of the formulation show that this product has its potential based on these two stability parameters.
Many methods have been used to evaluate and compare the fruits’ antioxidant activity due to the complexity of the substrates which need to be analyzed. The antioxidant capacity was mostly assessed using DPPH, which is one of the most commonly used tests for such an evaluation, since it is the method that determines the hydrophilic compounds [36].
Thus, the antioxidant capacity of the estimated Amazonian fruit based supplement showed to be 24.30±0.76 (IC 50μg.mL-1), demonstrating this value to be efficient (Table 1). Several studies indicate phenolic compounds to be the ones most responsible for the antioxidant activity in fruit, nevertheless, other studies suggest ascorbic acid to be the major contributor for this activity [13].
The determined amount of ascorbic acid showed an exceptional content of this nutrient, since it is superior to the nutritional recommendations for adults of both sexes [37]. Several studies have shown the bark of mature camu-camu to present a higher concentration of ascorbic acid and the presence of anthocyanin pigments. In this maturation process, the fruit reaches full growth and maximum edible quality, while still attached to the plant [38].
The antioxidant activity attributed to the ascorbic acid and polyphenols present in camu-camu, açaí and guaraná, associated with bioactive compounds, such as methylxanthines which are present in guarana, also contribute to its superior lipid profile, when these Amazonian fruits are consumed in the lyophilized form [4,12].
Studies performed on in natura Amazonian fruits, obtained the following results for anthocyanins, 42.2±17.0 and 111±30.4g.100-¹, AA 1.882±43.2 and 84.0±10g.100-¹ for camu-camu and açaí, respectively [9,32,39].
The present study ascertained the effect of the formulation on some enzymes such as α-amylase, α-glycosidase, and lipase. The tests that were performed showed there to be no inhibition of the digestive enzymes, and observed an activity index of less than 50%, maintaining the action androgens and estrogens hormones wich are produced locally in peripheral tissues, especially adipose tissue [40].
In addition, vitamins are organic compounds obtained through feeding which are necessary in the organism in small quantities and indispensable for the metabolic reactions in the interior of the cell, for normal growth and maintenance of health. Due to solubility in non-polar solvents, the fat-soluble vitamins are absorbed in the presence of bile acids and lipid digestion products. The amylase enzymes play a significant role in the digestive process, the function of which is to break sugars down, favoring postprandial glycemia [41].
During our study, the 37 days of oral administration of supplementation with Amazonian fruits and simvastatin showed that the treated rats presented no signs of an abnormality. No treatment-related variations in general health, body weight, food intake and clinical symptoms or basic observations of autonomic activity, were recorded. There was no systemic or local toxicity either. Macroscopic studies performed during necropsy showed the liver to be normal as well.
The group of animals that was treated daily with the simvastatin was used as a prediction parameter to verify that the supplement is functional, since this allopathic is prescribed for reducing the health risks derived from cardiovascular diseases by reducing blood cholesterol levels [42].
Notably, the group that received the highest concentration of the formulation (Figure 5E) was the one that showed the best result when compared to the control group (Figure 5A), thus observing the increase in MAT a phenomenon called transdifferentiation. Transdifferentiation is a direct conversion of unilocular adipocytes into multilocular adipocytes, and is regulated by the sympathetic nervous system with the branching of noradrenergic fibers in the parenchyma with a positive correlation between the density of these fibers and the number of multilocular adipocytes in most subcutaneous and visceral deposits [33].
In fact, browning is of healthy relevance because MAT prevents obesity and its related disorders [43,44]. Thus, darkening of adipose tissue could be an important strategy to prevent or treat obesity and its related complications. This plasticity could account for the normal coexistence of unilocular and multilocular adipocytes in most adipose tissue deposits, as for instance, when during chronic exposure to cold, unilocular adipocytes become multilocular adipocytes in order to contribute to thermogenesis and vice versa, and in case of chronic positive energy oscillation, multilocular adipocytes turn into unilocular ones so as to provide greater energy storage capacity [45]. In line with this theory, obese animals have a whitening of parts of the adipose organ, so these cells are able to reprogram their DNA as a way to distribute energy to functions essential for survival: thermogenesis or general metabolism [40].
Unilocular adipose tissue is responsible for most of the production of hormones whereas multilocular adipose tissue, is specialized in the production of heat (thermogenesis) and, therefore, actively participates in the regulation of body temperature [33].
As a result of studies, as well as the discovery of the unilocular adipose tissue's ability to secrete substances with important biological effects, great importance was attributed to its endocrine role. With the discovery of a wide range of the secreted bioactive proteins, called adipokines, a new concept on the biological function of this tissue has emerged, consolidating the idea of it not only being a supplier and stoker of energy, but a dynamic organ involved in metabolic and physiological processes, as well [44].
Recent studies have also suggested that hepatic steatosis is considered a silent disease and is becoming an ever more present liver disease in the general population. The main risk associated factors are obesity, dyslipidemias and diabetes mellitus. These predispose hypertension and metabolic syndrome [4].
In summary, our results suggest that a supplement based on the bioactive compounds present in the Amazonian fruits studied reduce serum levels of lipid profiles, and prevent hepatic fatty deposition effectively in an animal model. Therefore, the supplement is a potential food additive or pharmaceutical agent for treating or preventing hyperlipidemia. Clinical studies are being carried out to be used in all age groups since it has shown to preserve the functions of digestive enzymes, which are vital for metabolic balance.
Acknowledgement
The authors thank the Federal University of Amazonas and the Amazonian National Research Institute.
Funding
This study received no funding.
Disclosure
The authors have no conflicts of interest to declare.
Author’s Contribution
ASO, AASM, TCS, LBM, GAL, JFMB designed research, LDRA statistical analysis, FMAS data analysis, JFMB, ESL, RPC provided critical revision of the manuscript. Adele Salomão-Oliveira experimental studies, data acquisition, data analysis, editing and manuscript preparation.
Rosany Piccolotto Carvalho and Emerson Silva Lima contributed equally to this work.
References
- PAHO/WHO (2018) Comissão da OMS pede ação urgente contra doenças crônicas não transmissíve PAHO/WHO, Brazil.
- Ministério da Saúde (2017) Vigitel Brasil 2017: Vigilância de fatores de risco e proteção para doenças crônicas por inquérito telefônico: Estimativas sobre frequência e distribuição sociodemográfica de fatores de risco e proteção para doenças crônicas nas capitais dos 26 estados brasileiros e no Distrito Federal Em 2017.
- Faludi AA, Izar MCO, Saraiva JFK, Chacra APM, Bianco HT, et al. (2017) Atualização da Diretriz Brasileira de Dislipidemias e Prevenção da Aterosclerose. Arq Bras Cardiol 109: 1-76.
- Langley PC, Pergolizzi JV, Taylor R, Ridgway C (2015) Antioxidant and associated capacities of camu camu (Myrciaria dubia): A systematic review. J Altern Complement Med 21: 8-14.
- Yonekura L, Martins CA, Sampaio GR, Monteiro MP, César LAM, et al. (2016) Bioavailability of catechins from guaraná (Paullinia cupana) and its effect on antioxidant enzymes and other oxidative stress markers in healthy human subjects. Food Funct 7: 2970-2978.
- Klein T, Longhini R, Bruschi ML, Mello JCP (2009) Fitoterápicos: um mercado promissor. J Basic Appl Pharm Sci 30: 241-248.
- Salomão-Oliveira A, Costa SS, Marinho HA (2018) Metabolic syndrome: Intake of Camu-Camu (Myrciaria dubia (Kunth) McVaugh). International Journal of Food Science and Nutrition 3: 5-13.
- Salomão-Oliveira A, Lima ES, Marinho HA, Carvalho RP (2018) Benefits and effectiveness of using Paullinia cupana: A review article. Journal of Food and Nutrition Research 6: 497-503.
- Rufino MSM, Alves RE, Brito ES, Perez-Jimenez J, Saura-Calixto F, et al. (2010) Bioactive compounds and antioxidante capacitiesof 18 non-tradicional tropical fruits from Brazil. Food Chemistry 121: 996-1002.
- AOAC (2005) Official methods of analysis (18th edn). AOAC, Arlington, USA.
- Piga A, Catzeddu P, Farris S, Roggio T, Sanguinetti A, et al. (2005) Texture evaluation of Amaretti cookies during storage. Journal of Food Research and Technology 221: 387-391.
- Campos FM, Ribeiro SMR, Della Lucia CM, Stringheta PC, Pinheiro-Sant’Ana HM (2009) Optimization of methodology to analyze ascorbic acid and dehydroascorbic acid in vegetables. Química Nova 32: 87-91.
- Bonoli M, Verardo V, Marconi E, Carboni MF (2004) Antioxidant phenols in barley (Hordeum vulgare) flour: Comparative spectrophotometric study among extraction methods of free and bound phenolic compounds. J Agric Food Chem 52: 5195-5200.
- Singleton VL, Orthofer RRM, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology 299: 152-178.
- Fuleki T, Francis FJ (1968) Quantitative methods for anthocyanins. 2. Determination of total anthocyanin and degradation index for cranberry juice. Journal of Food Science 33: 78-83.
- Silva FMA, Hanna ACS, Souza AA, Filho FAS, CanhotoOMF, et al. (2019) Integrative analysis based on HPLC-DAD-MS/MS and NMR of bertholletia excelsa bark biomass residues: Determination of ellagic acid derivatives. J Braz Chem Soc 30: 830-836.
- Sousa CMM, Silva HR, Vieira GM, Ayres MCC, Costa CL, et al. (2007) Total phenolics and antioxidant activity of five medicinal plants. Quím Nova 30: 351-355.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, et al. (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26: 1231-1237.
- Rufino MSM, Alves RE, Brito ES, Morais SM, Sampaio CG, et al. (2006) Metodologia Científica: Determinação da Atividade Antioxidante Total em Frutas pelo Método de Redução do Ferro (FRAP).
- Camargo MCR, Toledo MCF (1998) Teor de cafeína em cafés Ciênc Tecnol Aliment 18: 421-424.
- Slanc P, Doljak B, Kreft S, Lunder M, Janes D, et al. (2009) Screening of selected food and medicinal plant extracts for pancreatic lipase inhibition. Phytother Res 23: 874-877.
- Subramaniam R, Asmawi MZ, Sadikun A (2008) In vitro α-glucosidase and α-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochim Pol 55: 391-398.
- Andrade-Cetto A, Becerra-Jiménez J, Cárdenas-Vázquez R (2008) Alfaglucosidase-inhibiting activity of some Mexican plants used in the treatment of type 2 diabetes. J Ethnopharmacol 116: 27-32.
- Wolf KL, Liu RH (2007) Cellular Antioxidant Activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J Agric Food Chem 55: 8896-8907.
- Nascimento OV, Boleti APA, Yuyama LKO, Lima ES (2013) Effects of diet supplementation with camu-camu (Myrciaria dúbia HBK Mc Vaugh) fruit in a rat model of diet-induced obesity. An Acad Bras Cienc 85: 355-363.
- Carlini E, Mendes FR (2011) Protocolos em psicofarmacologia comportamental: Um guia para a pesquisa de drogas com ação sobre o SNC, com ênfase nas plantas medicinais. São Paulo Editora Fap-Uniesp.
- Malone MH (1983) The pharmacological evalution of natural products-genetal and specific approaches to screening ethnopharmaceuticals. J Ethnopharmacol 8: 127-147.
- Liu ML, Jin YH, Li TH, Shi LH, Zhu BQ (2014) Effect of simvastatin on atherosclerosis and central aortic pressure in ApoE gene knockout mice. Zhejiang Da Xue Xue Bao Yi Xue Ban 43: 293-297.
- Bancroft JD, Stevens A (1982) Theory and practice oh histological techniques (2nd edn). Churchill Livingstone, London, UK.
- Gentili A, Caretti F, D'Ascenzo G, Marchese S, Perret D, et al. (2008) Simultaneous determination of water- soluble vitamins in selected food matrices by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Communications in Mass Spectrometry 22: 2029-2043.
- Chang CL, Wu RT (2011) Quantification of (+)-catechin and (−)-epicatechin in coconut water by LC–MS. Food chemistry 126: 710-717.
- Bresciani L, Calani L, Bruni R, Brighenti F, Del Rio D (2014) Phenolic composition, caffeine content and antioxidant capacity of coffee silverskin. Food research international 61: 196-201.
- Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, et al. (2010) The emergence of cold-induced brown adipocytes in mouse white fat depots isdetermined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 298: 1244-1253.
- Vasconcelos MAS, Filho ABM (2010) Técnico em Alimentos: Conservação de Alimentos. UFRPE/ CODAI 2010.
- Maeda RN, Pantoja L, Yuyama LKO, Chaar JM (2007) Estabilidade de ácido ascórbico e antocianinas em néctar de camu-camu (Myrciaria dúbia (H.B.K) Mc Vaugh). Ciência e Tecnologia de Alimentos 27: 313-316.
- Szabo M, Iditoiu C, Chambre D, Lupea A (2007) Improved DPPH determination for antioxidant activity spectrophotometric assay. Chemical Papers 61: 214-216.
- Institute of Medicine (2000) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary reference intakes: Applications in dietary assessment 2000. National Academy Press Washington DC, USA.
- Yuyama KA (2011) Cultura de camu-camu no Brasil. Revista Brasileira de Fruticultura 33: 335-690.
- Ribeiro PFA, Stringheta PC, Oliveira EB, Mendonça AC, Sant’Ana HMP (2016) Levels of vitamin C, β-carotene and minerals in camu-camu cultivated in different environments. Ciência Rural 46: 567-572.
- Rosenwald M, Perdikari A, Rulicke T, Wolfrum C (2013) Bi-directional interconversion of brite and white adipocytes. Nature cell biology 15: 659-667.
- Cozzolino SMF (2015) Biodisponibilidades de nutrientes (5th edn). Barueri, Brazil.
- Anvisa (2017) Agência Nacional de Vigilância Sanitár Pharlab Indústria Farmacêutica SA.
- Cypess AM, Kahn CR (2010) Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obes 17: 143-149.
- Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, et al. (2013) Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. The Journal of clinical investigation 123: 215-223.
- Cinti S (2011) Between brown and white: Novel aspects of adipocyte differentiation. Ann Med 43: 104-115.
Citation: Salomão-Oliveira A, Acho LDR, Magalhães AAS, Sumita TC, Matos LB, et al. (2021) Hypolipidemic Effect of Supplements Containing the Bioactive Compounds Found in Amazonian Fruits. J Food Sci Nutr 7: 095.
Copyright: © 2021 Adele Salomão-Oliveira, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

