
In Vitro Evaluation of Anthelmintic Interaction of Plant Species Combinations Putatively Containing Similar Bioactive Macromolecules in Sheep
*Corresponding Author(s):
Ignatius V NsahlaiDepartment Of Animal And Poultry Science, College Of Agriculture, Engineering And Sciences, 127 Rabie Saunders Building, SAEES, Private Bag X01, Scottsville 3209, PMB Campus Of UKZN, South Africa
Tel:+27 0332605067,
Fax:+27 0332605067
Email:fomslyw@gmail.com; nsahlaii@ukzn.ac.za
Abstract
Keywords
Bioactivity; Combination; Interaction; Macromolecules; Plant species; Synergy
INTRODUCTION
Non-chemical helminth control methods of this nature will serve as viable alternatives (options), which will reduce recurrent use of relevant chemical anthelmintics or reliance on a few. This, in effect, deters selection for resistant parasites, as has been strongly implicated in the current crisis [7]. Plants, by their very nature, contain a wide variety of bioactive macromolecules belonging to the same and/or different classes of compounds and can be likened to combined remedies setting precedence to anthelmintic therapy [8-12].
Combination anthelmintic therapy or prophylaxis has currently been adopted to retain high drug efficacy and simultaneously recycled those anthelmintics that have failed or currently exert relatively low efficacy [13-16]. Additionally, combination therapy widens spectrum of nematode parasite control within particular and different sites of infection in the gastrointestinal tract hosting them [17,18]. Gastrointestinal nematode infection of livestock poses a huge economic challenge to health and productivity of grazing-livestock globally, relative to those in confinement [7,19-22]. This problem has attained unprecedented level in small ruminants (goats and sheep), in addition to challenges of current control strategies [23]. Given the role of small ruminants as important source of wealth and animal protein to low resource households of Africa, Asia and most developing nations, any perturbation to their productivity or mortality resulting from infection by these parasites will have a huge setback on their income and livelihood [24]. Consistent and progressive failure of chemical anthelmintics, which has been the primary method of nematode parasite control from their inception, demand a review of previous and current modes of application and possibly a fundamental change to re-establish high efficacy [25-28]. Additionally, potential methods of improving ethno botanical anthelmintic efficacy will prove to be a very useful and crucial tool in the control process.
These changes are critical, because they will potentially arrest rapid development of resistant parasites and improve the efficacy span of anthelmintics in use, including subsequent candidates that will be developed. Additionally, research and development of new and more effective chemical candidates have similar high efficacy with preceding ones turned ineffective, as resistant nematode strains still emerge sooner or later [29,30]. Extensive research and development of new anthelmintic candidates could well be out of favour with the economic interest of major pharmaceutical industries, because of the sheer size of small ruminant industry that is mostly affected relative to other domestic ruminant species [7,30]. These challenges raise a lot of concerns, reaffirming dire need to conserve the efficacy of current anthelmintics and concurrently seek avenues of optimization. Existing control strategies not precluded, other viable and sustainable options should be explored, with the former ones serving as important platform for future research, innovation and development. Two principal methods of gastrointestinal parasite control of livestock have been implemented; external animal environmental control strategy that is prophylactic in nature, and internal gastrointestinal control using anthelmintic remedies that are either therapeutic or prophylactic. External strategies of control, some of which include grazing management, use of treated or conserved forages among others, seek to deter build-up of intermediate developing stages of parasites in the animal host environment [21,31]. Internal anthelmintic remedies are either chemical or bioactive principles that combat parasites within the host animal.
The later phase of gastrointestinal parasite control is critical to improve animal health and productivity. Wide-spread application of chemical anthelmintics has been fraught with wide ranging challenges, some of which include emergence of resistant strains to all classes of chemical anthelmintics, lodging of residues in animal products, environmental pollution by excreted un-metabolised chemical anthelmintics and induced resistance by rendering them unduly available to untargeted organisms [1-3,22,32-40]. Innovation of this method of control by adopting combination therapy has been another option of enhancing anthelmintic efficacy. It involves combined administration of two or more chemical anthelmintics, following waning efficacy of any one of them, thus altering and improving pharmacokinetic and pharmacodynamic activities of one or both [13,41-44]. These practices are commendable though resistant strains of gastrointestinal nematodes still emerge with time, when consistently used without alternating with other effective options. It is therefore critical to further explore and diversify other options, in view of attaining this goal. Besides, emergence of resistant gastrointestinal nematode parasites strain because of treatment with particular anthelmintics or anthelmintic combination(s), is irreversible [45]. Naturally, animals forage on various plants and may employ the activity of various bio-compounds in self-cure prevention. Similar practice can be employed using plants possessing/exerting anthelmintic activity.
Combination anthelmintic phytotherapy has been used for a long time, without any sound scientific basis of the bioactive principles involved, and interactions leading to improved efficacy. It is hypothesized that combination of plant species crude extract exerting anthelmintic activity will produce no synergistic or antagonistic effects, and correspondingly observed efficacy, synergy and antagonism will not relate to plant secondary metabolites including alkaloids, condensed tannins and flavonoid content of plant species. The specific objective was to evaluate and identify plant extracts with potential of being used in combination to develop a more effective livestock nematode control remedy. This study also elucidated the quantitative contribution of alkaloids, condensed tannins and flavonoids of component plant species to anthelmintic efficacy.
MATERIALS AND METHODS
Collection of vegetative plant material and processing of crude extracts
- Alkaloids and condensed tannins containing plant species which included Crinum macowanii, Gunnera perpensa, Nicotiana tabacum, Sarcostemma viminale, Vernonia amygdalina, Zingiber officinale, Zizyphus mucronata and Aloe vanbalenii [40,46-56].
- Flavonoids containing plant species comprised of Trema orientalis, Urtica dioica and Zanthozylum capense [53,55,57,58]
- Proteinases and nitrogenous compound containing plant species were made of, Allium cepa, Ananas comosus, Bidens pilosa, Carica papaya and Ricinus communis [37,59-66].
|
Plant species |
Family |
Common name |
|
Allium cepa |
Amaryllidaceae |
Common onion |
|
Aloe vanbalenii |
Aloeaceae |
Van Balen’s aloe |
|
Ananas comosus |
Bromeliaceae |
pineapple |
|
Bidens pilosa |
Asteraceae |
Black-jack |
|
Carica papaya |
Caricaceae |
Pawpaw |
|
Crinum macowanii |
Amaryllidaceae |
River lily |
|
Gunnera perpensa |
Gunneraceae |
River pumkin |
|
Nicotiana tabacum |
Solanaceae |
Tobacco |
|
Ricinus communis |
Euphorbiaceae |
Castor oil plant |
|
Sarcostemma viminale |
Asclepiadaceae |
Caustic vine |
|
Trema orientalis |
Cannabaceae |
Pigeon wood |
|
Urtica dioica |
Urticaceae |
Stinging nettle |
|
Vernonia amygdalina |
Asteraceae |
Bitterleaf |
|
Zanthozylum capense |
Rutaceae |
Small knobwood |
|
Zingiber officinale |
Zingiberaceae |
Ginger |
|
Zizyphus mucronata |
Rhamnaceae |
Buffalo thorn |
For each plant, fresh vegetative material was collected, washed, chopped and processed as described in the current section. Those with large and long leaves, were first air dried to reduce moisture content and subsequently oven dried (Oven mark; LABCON, Model 5SOEIB, Maraisburg 1700) to constant weight at 60°C. Oven-dried material of each plant species was milled using an electric centrifuge mill (RETSCH, GmbH and Co.KG, 5657 HAANI, West Germany), fine enough to pass through a 1-mm sieve. Milled plant samples were then put into air-tight labeled plastic containers and stored in boxes, away from light and moisture at room temperature.
A milled sample (4g) Dry Matter (DM) of each plant species was weighed into labeled thimble, fitted into distillation column and extracted in 70% ethanol over a heating unit (GERHARDT BONN, App. Nr 450893). The extraction process was considered complete when the solvent in the thimble carrying unit was apparently free of any coloration. At which point, plant crude extracts were obtained from bottles into which they were drained and concentrated. Bottles, which fell below the 100 ml mark, were made up to standardized volume by adding solvent (70% ethanol) and sealed with parafilm® (PARAFILM®AMERICAN NATIONAL CanTM, Neenah, W154956). They were packaged into boxes and stored in a fridge for in vitrodosing of mixed cultured isolated nematode L3 larvae.
Quantitative analysis of alkaloids, flavonoids and condensed tannins in plant samples
Flavonoids content of selected plant vegetative material was determined following [68]. Ten grams (10g) of oven-dried plant material was milled to pass through a 1-mm sieve, weighed into a 250 ml sterile beaker, and 100ml of 80% aqueous methanol added to it. The content was allowed to stand for 10 hours at room temperature, while being stirred intermittently with a magnetic stirring bar over a magnetic rotor without heat. Each solution was filtered individually through WHATMANTM No 42 filter paper. The filtrate of each sample was transferred into pre-weighed 250ml conical flask and evaporated to dryness in a water bath at constant temperature (80°C). Flasks and their contents were allowed to cool and subsequently placed in the desiccators for one hour to rid them of any moisture. Each of them was weighed, and the weight of the sterilized conical flask deducted from that of flask and flavonoid. The difference was computed as a percentage of flavonoid content of different species.
Condensed tannins were analysed following HCl-Butanol proanthocyanidin assay as leucocyanidin equivalent [69,70]. By which, one and a half grams (1.5g) of this material was weighed into pre-weighed filter paper (WHATMANTM, number 1, diameter 110mm, Cat number 1001 - 110, GE Healthcare UK limited, Amersham Place, Little Chalfont, Buckinghamshire. HP7, 9NA, UK, and Made in China) and Sohxlet extracted in 1% glacial in petroleum ether to rid them of pigments and fats that could interfere with quantitative determination of condensed tannins. It should be noted that the addition of glacial to petroleum ether serves as antioxidant, and prevents condensed tannins from being oxidized and bound to vegetative material. Weights of maximum 0.2001 g samples of the different plant species were measured into 100ml plastic centrifuge tubes, and 10ml of 70% aqueous acetone added to extract condensed tannins. Centrifuge tubes and their contents were Vortex mixed and placed in an ice bath. Samples were subjected to ultrasonic treatment for 3 minutes in ice cold water and vortex mixed intermittently for 12 minutes, resulting to 4 ultrasonic treatments in all. The content was centrifuged at 5000 rotations per minute (rpm) for 20 minutes at 4°C, and supernatant carefully collected in a glass test tube and stored on ice. Appropriate dilutions of tannin extracts with 70% aqueous acetone were made. Butanol reagent of volume 6 ml (950 ml of butanol and 50 ml of HCl 37% ) and 0.2 ml of ferric reagent (16.6 ml of concentrated HCl 37% diluted to 100 ml with water to make 2 M HCl and 2 g of ammonium ferric sulphate dissolved in it) was added to the tubes and vortex mixed. Tubes were covered and placed in a heating bath adjusted to between 96-100°C for 60 minutes. At the end of the incubation, they were cooled and absorbances measured using BECKMAN DU®640 Spectrophotometer at visible wavelength of light 550 nm. From each of these absorbances red, was deducted that of an unheated mixture (blank). The method allows for appropriate absorbances between 0.30 and less than or equal to 0.60 to be considered stable and most appropriate. Percentage condensed tannins in each of the plant samples were computed following the formula below:
Percentage condensed tannins in dry matter= A550nm x 78.26 x D/% dry matter, where: A550nm= Absorbance at 550 nm; 78.26 = Accumulative factor taking into account: extinction coefficient of leucocyanidin, mass of sample (200g) and other factors except dilution; D=Dilution factor.
Extraction and in vitro dosing of sheep dung
Experimental design, computation of anthelmintic plant species interaction resulting to either synergistic or antagonistic effects and statistical analysis
Two approaches were used to compute additive and synergistic anthelmintic effects resulting from in vitro combination therapy. In the first method, expected combined efficacies of any pair of plant species ‘a’ and ‘b’ were computed (a+b)/2 and subsequently deducted from their observed combined efficacy to yield simple synergy. Positive differences measured synergistic effects, whereas negative differences measured antagonistic effects. In the second method, expected efficacy of plant species combination was estimated following Webb’s fractional product method [72]. Following this method, if the efficacies of two plant species “A” and “B” represented by “a” and “b” proportion of worms killed, then expected efficacy of combinations assuming additive effect is computed thus: Efficacy (A+B) = 1 - ((1 - a) x (1 - b)). Synergistic effect is considered to have occurred when the response of combined administration is greater than additive.
Data collected in each of these three experiments was analysed following General Linear Model (GLM) of [73]. Pearson correlation was used to seek possible relationship between anthelmintic efficacy on the one hand and each of alkaloids, flavonoids and condensed tannins as primary putative anthelmintic macromolecules. Additionally, multiple regression analysis was run to seek explanations of the role of various variables including alkaloids, flavonoids and condensed tannins to observed trends of anthelmintic efficacy. Mean separation was done using Student Neuman Keul’s statistic, aided by [73].
RESULTS
|
|
Combined treatment (A + B) |
Observed (A) % |
Observed (B) % |
Expected |
Observed |
Webb’s efficacy % |
Simple synergy |
Webb synergy (Observ. –Webb) |
Additive (A) or synergistic (S) |
|
Tan/Tan |
Crin-Alo |
91.2±0.13 |
91.2±0.13 |
91.2 |
93.2±0.05 |
99.2 |
2.0±0.05 |
-6.0±0.05 |
A |
|
Crin-Gun |
91.2±0.13 |
82.4±0.13 |
86.8 |
93.3±0.05 |
98.5 |
6.5±0.05 |
-5.1±0.05 |
A |
|
|
Crin-Nic |
91.2±0.13 |
91.2±0.13 |
91.2 |
93.2±0.54 |
99.2 |
2.0±0.05 |
-6.0±0.54 |
A |
|
|
Crin-Sarc |
91.2±0.13 |
82.4±0.13 |
86.8 |
90.0±0.05 |
98.5 |
3.2±0.05 |
-8.5±0.05 |
A |
|
|
Crin-Vern |
91.2±0.13 |
82.5±0.13 |
86.9 |
85.4±0.05 |
98.5 |
-1.4±0.05 |
-13.0±0.05 |
A |
|
|
Crin-Zin |
91.2±0.13 |
82.4±0.13 |
86.8 |
93.8±0.05 |
98.5 |
7.0±0.05 |
-4.7±0.05 |
A |
|
|
Crin-Ziz |
91.2±0.13 |
91.2±0.13 |
91.2 |
95.7±0.05 |
99.2 |
4.5±0.05 |
-3.5±0.05 |
A |
|
|
Gun-Alo |
82.4±0.13 |
91.2±0.13 |
86.8 |
96.7±0.05 |
98.5 |
9.9±0.05 |
-1.8±0.05 |
A |
|
|
Gun-Nic |
82.4±0.13 |
91.2±0.13 |
86.8 |
100.0±0.05 |
98.5 |
13.2±0.05 |
1.5±0.05 |
S |
|
|
Gun-Sarc |
82.4±0.13 |
82.4±0.13 |
82.4 |
90.1±0.05 |
96.9 |
7.7±0.05 |
-6.8±0.05 |
A |
|
|
Gun-Vern |
82.4±0.13 |
82.5±0.13 |
82.5 |
96.7±0.05 |
96.9 |
14.2±0.05 |
-0.3±0.05 |
A |
|
|
Gun-Zin |
82.4±0.13 |
82.4±0.13 |
82.4 |
93.3±0.05 |
96.9 |
10.9±0.05 |
-3.6±0.05 |
A |
|
|
Gun-Ziz |
82.4±0.13 |
91.2±0.13 |
86.8 |
96.3±0.05 |
98.5 |
9.5±0.05 |
-2.1±0.05 |
A |
|
|
Nic-Alo |
91.2±0.13 |
91.2±0.13 |
91.2 |
99.4±0.05 |
99.2 |
8.2±0.05 |
0.2±0.05 |
S |
|
|
Nic-Sarc |
91.2±0.13 |
82.4±0.13 |
86.8 |
83.1±0.05 |
98.5 |
-3.7±0.05 |
-15.3±0.05 |
A |
|
|
Nic-Vern |
91.2±0.13 |
82.5±0.13 |
86.9 |
96.7±0.05 |
98.5 |
9.8±0.05 |
-1.8±0.05 |
A |
|
|
Nic-Zin |
91.2±0.13 |
82.4±0.13 |
86.8 |
88.3±0.05 |
98.5 |
10.9±0.05 |
-10.1±0.05 |
A |
|
|
Nic-Ziz |
91.2±0.13 |
91.2±0.13 |
91.2 |
81.6±0.05 |
99.2 |
-9.6±0.05 |
-17.7±0.05 |
A |
|
|
Sarc-Alo |
82.4±0.13 |
91.2±0.13 |
86.8 |
92.9±0.05 |
98.5 |
6.1±0.05 |
-6.6±0.05 |
A |
|
|
Sarc-Vern |
82.4±0.13 |
82.5±0.13 |
82.5 |
97.6±0.05 |
96.9 |
15.2±0.05 |
0.7±0.05 |
S |
|
|
Sarc-Ziz |
82.4±0.13 |
91.2±0.13 |
86.8 |
97.6±0.05 |
98.5 |
10.8±0.54 |
-0.9±0.05 |
A |
|
|
Vern-Alo |
82.5±0.13 |
91.2±0.13 |
86.9 |
97.6±0.05 |
98.5 |
10.8±0.05 |
-0.8±0.05 |
A |
|
|
Vern-Zin |
82.5±0.13 |
82.4±0.13 |
82.5 |
99.9±0.05 |
96.9 |
17.5±0.05 |
3.0±0.05 |
S |
|
|
Vern-Ziz |
82.5±0.13 |
91.2±0.13 |
86.9 |
97.4±0.05 |
98.5 |
10.5±0.05 |
-1.1±0.05 |
A |
|
|
Zin-Alo |
82.4±0.13 |
91.2±0.13 |
86.8 |
99.9±0.05 |
98.5 |
13.1±0.05 |
1.4±0.05 |
S |
|
|
Zin-Ziz |
82.4±0.13 |
91.2±0.13 |
86.8 |
99.9±0.05 |
98.5 |
13.1±0.05 |
1.5±0.05 |
S |
|
|
Ziz-Alo |
82.4±0.13 |
91.2±0.13 |
91.2 |
100.0±0.05 |
99.2 |
8.8±0.05 |
0.8±0.05 |
S |
|
|
Flav/ |
Trem-Urt |
73.5±0.13 |
82.5±0.13 |
78.0 |
100.0±0.54 |
95.4 |
22.0±0.54 |
4.6±0.54 |
S |
|
Trem-Zan |
73.5±0.13 |
82.5±0.13 |
78.0 |
85.2±0.54 |
95.4 |
7.2±0.54 |
-10.1±0.54 |
A |
|
|
Urt-Zan |
82.5±0.13 |
82.5±0.13 |
82.5 |
97.6±0.54 |
96.9 |
15.1±0.54 |
0.7±0.54 |
S |
|
|
Prott |
All-Ana |
82.3±0.13 |
91.2±0.13 |
86.8 |
97.6±0.10 |
98.4 |
10.9±0.10 |
-0.8±0.10 |
A |
|
All-Bid |
82.3±0.13 |
73.5±0.13 |
77.9 |
97.6±0.10 |
95.3 |
19.7±0.10 |
2.3±0.10 |
S |
|
|
All-Car |
82.3±0.13 |
91.2±0.13 |
86.8 |
99.3±0.10 |
98.4 |
12.5±0.10 |
0.8±0.10 |
S |
|
|
All-Ric |
82.3±0.13 |
73.5±0.13 |
77.9 |
97.6±0.10 |
95.3 |
19.7±0.10 |
2.3±0.10 |
S |
|
|
Ana-Bid |
91.2±0.13 |
73.5±0.13 |
82.4 |
97.6±0.10 |
97.7 |
15.2±0.10 |
-0.1±0.10 |
A |
|
|
Ana-Car |
91.2±0.13 |
91.2±0.13 |
91.2 |
100.0±0.10 |
99.2 |
8.8±0.10 |
0.8±0.10 |
S |
|
|
Ana-Ric |
91.2±0.13 |
73.5±0.13 |
82.4 |
97.5±0.10 |
97.7 |
15.1±0.10 |
-0.2±0.10 |
A |
|
|
Bid-Car |
73.5±0.13 |
91.2±0.13 |
82.4 |
100.0±0.10 |
97.7 |
17.7±0.10 |
2.3±0.10 |
S |
|
|
Bid-Ric |
73.5±0.13 |
73.5±0.13 |
73.5 |
92.8±0.10 |
93.0 |
19.3±0.10 |
-0.2±0.10 |
A |
|
|
Car-Ric |
91.2±0.13 |
73.5±0.13 |
82.4 |
98.8±0.10 |
97.7 |
16.4±0.10 |
1.1±0.10 |
S |
|
|
Plant species |
n |
Alkaloids (gDM/Kg) |
n |
Cond. Tannins (gDM/Kg) |
n |
Flavonoids (gDM/Kg) |
|
Alkaloids ad condensed tannins |
|
|
|
|
|||
|
|
Crinum m. |
2 |
20.9±1.10A |
6 |
5.5±1.28A |
2 |
117.9±1.75B |
|
|
Gunnera p. |
2 |
44.4±15.20A |
5 |
7.6±1.30B |
2 |
26.0±2.60A |
|
|
Nicotiana t. |
2 |
37.1±3.20A |
5 |
6.4±1.42B |
2 |
202.6±0.75A |
|
|
Sarcostema v. |
2 |
46.7±8.50A |
2 |
2.8±0.01B |
2 |
117.0±2.76B |
|
|
Vernonia a. |
2 |
42.4±8.20A |
6 |
3.4±0.63B |
2 |
125.0±13.57B |
|
|
Zingiber o. |
2 |
48.3±4.50A |
6 |
3.4±0.55B |
2 |
172.1±17.60A |
|
|
Zizyphus m. |
2 |
30.6±0.68A |
6 |
13.7±1.99B |
2 |
124.3±10.67B |
|
Flavonoids |
|
|
|
||||
|
|
Trema o. |
2 |
72.5±13.80A |
3 |
11.5±2.14A |
2 |
207.5±1.66A |
|
|
Urtica d. |
2 |
23.6±17.90A |
6 |
11.2±1.61A |
2 |
138.6±6.63B |
|
|
Zanthozylum c. |
2 |
16.3±1.22A |
6 |
3.9±1.47B |
2 |
129.1±16.01B |
|
Proteases and or nitrogen compounds |
|
|
|
||||
|
|
Allium c. |
2 |
5.7±0.30A |
6 |
4.7±0.97A |
2 |
550.4±25.42A |
|
|
Ananas c. |
2 |
47.5±6.70A |
6 |
4.4±0.75A |
2 |
133.5±5.15B |
|
|
Bidens p. |
2 |
39.5±6.10A |
6 |
5.9±1.09A |
2 |
163.5±1.92B |
|
|
Carica p. |
2 |
40.5±6.10A |
4 |
2.6±0.76A |
2 |
167.7±12.38B |
|
|
Ricinus c. |
2 |
43.0±4.80A |
6 |
4.4±1.56A |
2 |
149.6±10.27B |
Sub-experiment one (combined efficacies of plant species containing alkaloids and tannins)
Alkaloids, condensed tannins and flavonoids were identified and their concentrations evaluated in these plant species. Alkaloid contents were similar (P=0.304), with mean concentration of 38.6±0.68 g/KgDM (Table 3). Concentrations of condensed tannins of different plant species were different (P<0.0001), with mean content of 6.0±0.13 g/KgDM. The trend of tannin content was: Z. mucronata>G. perpensa>N. tabacum>C. macowanii>V. amygdalina>Z. officinale>S. viminale (Table 3). Correspondingly, flavonoid contents were different (P= 0.0006), and had mean content 152.1±0.74 g/KgDM. The trend of flavonoid content was thus: N. tabacum>Z. officinale>V. amygdalina>Z. mucronata>C. macowanii>S. viminale>G. perpensa (Table 3). The order of macro biochemical content for all plant species in this group was, flavonoids> alkaloids>condensed tannins. There was no correlation between combined efficacy and any of alkaloids (r=0.1458; P=0.2543), condensed tannins (r= 0.0059; P=0.9637) or flavonoids (r= -0.0293; P=0.8199). Alkaloids, condensed tannins and flavonoids were all poor predictors of combined efficacy for plant species possessing alkaloids and tannins in a multi-regression analysis as no variables met the criterion of P=0.15.
Sub-experiment two (combined efficacies of plant species containing flavonoids)
Quantitatively, content of condensed tannins, alkaloids and flavonoids (Table 3) of these plant species had various relationships. Condensed tannin content were different (P=0.0089) among plant species, with a mean 8.3±0.91 g/KgDM, whereas alkaloid content were not different (P=0.07), and had mean 37.5±1.93 g/KgDM. Additionally, flavonoid content of these plants were different (P=0.0211) among them, with mean 156.4±1.78 g/KgDM. There was no discernible association between anthelmintic efficacy and any of alkaloids (r= -0.1411; P=0.7173), condensed tannins (r=0.3361; P=0.3765) or flavonoid (r= -0.1457; P=0.7084) content of these plant species. Multiple regression analysis of alkaloids, condensed tannins and flavonoid content as predictors of combined efficacy was not significant, as none entered the model at P= 0.15.
Sub-experiment three (combined efficacies of plant species containing proteases and nitrogen compounds)
Alkaloid contents were different (P=0.0135) and had mean 35.2±0.78 g/KgDM (Table 3), whereas condensed tannin contents were similar (P=0.4312), with mean 4.5±0.46 g/KgDM. Flavonoid contents were also different (P<0.0001) with mean 232.9±1.24 g/kgDM. There was no correlation between any of alkaloids (r= -0.02774; P= 0.8843), condensed tannins (r= -0.3071; P=0.0987) or flavonoids (r=0.0359; P=0.8505) and observed efficacy. A multi-regressions of alkaloids, condensed tannins and flavonoids as predictors of combined anthelmintic efficacy also gave no relationship. Overall trend of concentration for all three biochemical compounds was flavonoids> alkaloids> condensed tannins (Table 3).
Relationship between simple and Webb synergy in sub experiments 1, 2 and 3
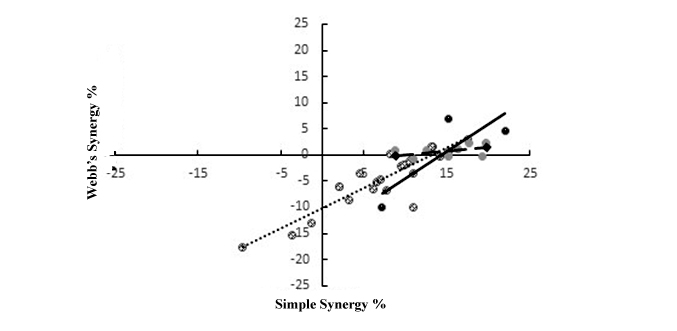 Figure 1: Scattered plot of interaction between simple and Webb’s synergies for combinations involving tannin/tannin (dash trend line), flavonoid /flavonoid (solid trend line) and proteases (broken trend line) containing plant species.
Figure 1: Scattered plot of interaction between simple and Webb’s synergies for combinations involving tannin/tannin (dash trend line), flavonoid /flavonoid (solid trend line) and proteases (broken trend line) containing plant species.DISCUSSION
In all three sub experiments, alkaloids, condensed tannins and flavonoids were quantitatively evaluated for all plant species. Given that empirical evidence closely links alkaloids, condensed tannins and flavonoids to exerting anthelmintic activity, a natural expectation would be some level of association of these macromolecules with anthelmintic efficacy in plant species in which they occur [36,46,51,75-85]. Contrary to this expectation, there was no discernible correlation of all three macromolecular compounds with anthelmintic efficacy. In combinations, the collective activity of these macromolecules and others that might not have been identified may be responsible for the observed activity. Additionally, anthelmintic potency of these principles put together, may far exceed or shield that of individual macromolecules including alkaloids, condensed tannins and flavonoids. In attempt to formulate some predictive measure of combined anthelmintic efficacy or interchangeably observed efficacy based on the contribution of alkaloids, condensed tannins and flavonoids in the current study, a multi-regression analysis failed to prove any contribution. This reaffirms collective rather than strong individual contribution of various anthelmintic principles to efficacy, helping in the proposition of very high dosages of these plant species.
Plant species containing alkaloids generally have a wide variety of these macromolecules and/or other related biochemical’s with different chemical structures, some of which are isomers and others have different molecular weights [86-88]. A similar pool of various types of tannins occurred in plant species possessing condensed tannins [89-91]. Flavonoid, containing plant species likewise have a wide variety of different flavonoids and other biochemical’s [88,92], and sometimes different isomers of the same flavonoid occur in the same and also in different plant species [88]. Similarly, the biochemical content of proteases and nitrogen compounds in plant species containing this macromolecular class would have been structurally diverse, in addition to other related anthelmintics [93]. This structural biochemical diversity of alkaloids and tannins, flavonoids and, proteases and nitrogen compounds in different plant species confers on them different properties and biochemical activities [88,89,91-93]. Therefore, a wider pool of biochemical compounds in combinations, different interactions among them or pharmacodynamic activities, and by inference various pharmacokinetic activities [94]. This would occur first, within a similar class of anthelmintic bioactive pool such as alkaloids, condensed tannins and flavonoids in plant species and cumulatively in different plant species constituting various combinations (combined biochemical pool), yielding improved efficacy as targeted and observed in the current study [95,96]. This further strengthens regards for collective rather than for individual anthelmintic activity.
Based on the current study, a much more reduced combined dose is required to properly address synergistic activity. While the other parameters show that there is potentially greater potency in combined anthelmintic phytotherapy, synergy is more negative than positive. Relationship between simple and Webb synergies has highlighted some combinations to have exceedingly higher efficacy than would have resulted from any one of the component plant species individually at the same concentration. It is therefore imperative to ascertain this synergistic interaction in vivo, as these combinations hold a huge potential in nematode control programs. Improved observed efficacy in the current study is generally in accord with its primary objective. Antagonistic and synergistic activities as observe from both correlation matrix and multiregression occur in plant species exerting anthelmintic activities and other relevant antiparasitic activities. The biological activity identified in plant species suggest that various modulating chemical activities occur within each plant species or combinations to enable them exercise their activity without causing harm to livestock that is treated. Additionally, different types of active compounds may occur in the same plant species (Table 1.2) or in different plant species, conferring on them broad spectrum nematode parasite control capacity [95,97]. This biochemical nature, inherently accords advantage to anthelmintic activity. Combination therapy, therefore, yields multicomponent active principle, which exerts different modes of biochemical activity, leaving nematode parasites insufficient capacity to develop resistance against all of them relative to single plant species treatment [74].
Though combination brings together a variety of bioactive anthelmintic principles, in nature, individual plant species harbour different primary biochemical’s including alkaloids, condensed tannins, flavonoids and other compounds (not determined in this study) in different concentrations. Naturally, anthelmintic biochemical variety exists at the level of individual plant species. Some of the bioactive anthelmintic principles including alkaloids, tannins, flavonoids and terpenes among others, are plant secondary metabolites, which when present in diets beyond certain thresholds negate their beneficial effects [98,99]. A variety of these macromolecules in diets have been suggested to mitigate or modulate the negative effects of individual plant species, implicitly by dilution or counteraction of deleterious effects of each other. These interactive and modulatory effects enhance the nutritional and curative benefits to animal production and health [98]. In the same vein, combinations of plant species exerting anthelmintic activity that result to enhanced activity greater than that of individual component species, is an expression of the merits of a much broader positive interaction. Measurable and relatively enhanced anthelmintic efficacy emanating from combination of different plant species has been adopted from its application in chemical or orthodox anthelmintic combination [72].
Improved activity from combination anthelmintic phytotherapy will result to more potent activity relative to that of individual component species, and similar to what obtains in combination chemical anthelmintic therapy [74]. This heightened activity has the likelihood of complete parasite elimination at contact or parasite/biochemical interaction. Remnants of surviving parasites that would not have had any contact or interaction with combined extract will retain their integral vulnerability to subsequent dosing. This is typical of the concept of refugia where in, the surviving portion of parasites in a herd of animals is made to have absolutely no contact or interaction with anthelmintic drug in use, while those with any contact are killed [22]. Efficacious drug disposition of this nature is critical in livestock nematode control programs because of prevention of selection for resistant parasites and build-up of resistant alleles that usually lead to drug failure.
Among different plant species possessing similar biochemical class but with different structural formulae, some of them may exert anthelmintic activity and others not, suggesting that all plant species possessing these tagged bioactive principle classes may not be necessarily anthelmintic in nature, or may not exert this activity to the same extent when it exists [100,101]. Additionally, plant species content of any of alkaloids, condensed tannins and flavonoids does not conclusively translate to anthelmintic biological activity, because not all macromolecules exert this activity [100]. This raises the need for a much finer biochemical profiling, leading to extensive identification of all potential contributors to this anthelmintic trait. There is need to ascertain advantages of combination anthelmintic phytotherapy or chemotherapy offered over either of them individually. The genetic basis of combination anthelmintic phytotherapy or chemotherapy is crucial in nematode parasite control programs. Chemical anthelmintic therapy presents peculiar cases of anthelmintic resistance, wherein selection for resistance against an anthelmintic does not necessarily affect others in the same or different groups because of different mechanisms of action [102,103]. Independent selection for resistance ties with the genetic control of this trait, which is in turn controlled by different alleles [14]. Combination anthelmintic therapy renders genetic selection for resistance difficult, though not impossible, as many alleles will have to be involved in the process. Combination therapy, therefore, affords the opportunity to recycle anthelmintics, which otherwise would have been ineffective or exerting low efficacy individually, the opportunity to exert acceptable levels of efficacy because of the genetic disposition towards this trait. Additionally, component anthelmintics in combination can influence and radically improve pharmacokinetic and pharmacodynamic interactions of the duo resulting to additive and synergistic effects on efficacy [15]. Similarly, plant bioactive anthelmintic principles constitute a pool of various biochemical classes and types of macromolecules, rendering genetic control much more intricate and complicated. Plants by their biological nature require huge genetic alterations in parasites to take place in order for parasites to become resistant; which is farfetched and inherently advantageous in livestock nematode parasite control programs. Ethno veterinary phytochemical combination anthelmintic therapy therefore constitutes an important option in parasite control that should be accorded adequate attention in research, development and treatment.
CONCLUSION
ACKNOWLEDGEMENT
CONFLICT OF INTEREST
REFERENCES
- Terrill TH, Kaplan RM, Larsen M, Samples OM, Miller JE, et al. (2001) Anthelmintic resistance on goat farms in Georgia: efficacy of anthelmintics against gastrointestinal nematodes in two selected goat herds. Vet Parasitol 97: 261-268.
- Jackson F, Bartley D, Bartley Y, Kenyon F (2009) Worm control in sheep in the future. Small Ruminant Research 86: 40-45.
- Kaplan RM, Vidyashankar AN (2012) An inconvenient truth: global worming and anthelmintic resistance. Vet Parasitol 186: 70-78.
- Geerts S, Gryseels B (2000) Drug resistance in human helminths: current situation and lessons from livestock. Clin Microbiol Rev 13: 207-222.
- Kaplan RM (2004) Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol 20: 477-481.
- Ketzis JK,Vercruysse J, Stromberg B, Larsen M, Athanasiadou S, et al. (2006) Evaluation of efficacy expectations for novel and non-chemical helminth control strategies in ruminants. Vet Parasitol 139: 321-335.
- Besier B (2007) New anthelmintics for livestock: the time is right. Trends Parasitol 23: 21-24.
- Klongsiriwet C, Quijada J, Williams AR, Mueller-Harvey I, Williamson EM, et al. (2015) Synergistic inhibition of Haemonchus contortus exsheathment by flavonoid monomers and condensed tannins. Int J Parasitol Drugs Drug Resist 5: 127-134.
- Sajid M, McKerrow JH (2002) Cysteine proteases of parasitic organisms. Mol Biochem Parasitol 120: 1-21.
- Choi S, Chung MH (2003) A review on the relationship between aloe vera components and their biologic effects. Seminars in Integrative Medicine 1: 53-62.
- Makkar HPS (2006) Chemical and biological assays for quantification of major plant secondary metabolites. BSAP Occasional Publication 235-249.
- Hoste H, Torres-Acosta JF, Alonso-diaz MÁ, Brunet S, Sandoval-Castro C, et al. (2008) Identification and validation of bioactive plants for the control of gastrointestinal nematodes in small ruminants. Trop Biomed 25: 56-72.
- Entrocasso C, Alvarez L, Manazza J, Lifschitz A, Borda B, et al. (2008) Clinical efficacy assessment of the albendazole-ivermectin combination in lambs parasitized with resistant nematodes. Vet Parasitol 155: 249-256.
- Bartram DJ, Leathwick DM, Taylor MA, Geurden T, Maeder SJ (2012) The role of combination anthelmintic formulations in the sustainable control of sheep nematodes. Vet Parasitol 186: 151-158.
- Lanusse C, Alvarez L, Lifschitz A (2014) Pharmacological knowledge and sustainable anthelmintic therapy in ruminants. Vet Parasitol 204: 18-33.
- Lanusse C, Lifschitz A, Alvarez L (2015) Basic and clinical pharmacology contribution to extend anthelmintic molecules lifespan. Vet Parasitol 212: 35-46.
- Athanasiadou S, Kyriazakis I, Jackson F, Coop RL (2001) Direct anthelmintic effects of condensed tannins towards different gastrointestinal nematodes of sheep: in vitro and in vivo Vet Parasitol 99: 205-219.
- Marley CL, Cook R, Keatinge R, Barrett J, Lampkin AH (2003) The effect of birdsfoot trefoil (Lotus corniculatus) and chicory (Cichorium intybus) on parasite intensities and performance of lambs naturally infected with helminth parasites. Vet Parasitol 112: 147-155.
- Vercruysse J, Charlier J, Dorny P, Claerebout E (2006) Diagnosis of Helminth Infections in Cattle: Were we Wrong in the Past? In: proceedings of the 24th world buiatrics congress Nice, Merelbeke, Belgium.
- Waller PJ (1997) Sustainable helminth control of ruminants in developing countries. Vet Parasitol 71: 195-207.
- Athanasiadou S, Houdijk J, Kyriazakis I (2008) Exploiting synergisms and interactions in the nutritional approaches to parasite control in sheep production systems. Small Ruminant Research 76: 2-11.
- Sutherland IA, Leathwick DM (2011) Anthelmintic resistance in nematode parasites of cattle: a global issue? Trends Parasitol 27: 176-181.
- Jackson F, Coop RL (2000) The development of anthelmintic resistance in sheep nematodes. Parasitology 120: 95-107.
- Perry BD, Randolph RF, McDermott JJ, Sones KR, Thornton PK (2002) Investing in Animal Health Research to Alleviate Poverty. International Livestock Research Institute, Nairobi, Kenya, Pg no: 148.
- Waller PJ1 (1997) Anthelmintic resistance. Vet Parasitol 72: 391-405.
- Jackson F, Miller J (2006) Alternative approaches to control--quo vadit? Vet Parasitol 139: 371-384.
- Kaminsky R, Ducray P, Jung M, Clover R, Rufener L, et al. (2008) A new class of anthelmintics effective against drug-resistant nematodes. Nature 452: 176-180.
- López-Aroche U, Salinas-Sánchez DO, Mendoza de Gives P, López-Arellano ME, Liébano-Hernández E, et al. (2008) In vitro nematicidal effects of medicinal plants from the Sierra de Huautla, Biosphere Reserve, Morelos, Mexico against Haemonchus contortus infective larvae. J Helminthol 82: 25-31.
- Coop RL, Kyriazakis I (2001) Influence of host nutrition on the development and consequences of nematode parasitism in ruminants. Trends Parasitol 17: 325-330.
- Várady M, Papadopoulos E, Dolinská M, Königová A (2011) Anthelmintic resistance in parasites of small ruminants: sheep versus goats. Helminthologia 48: 137-144.
- Fleming SA, Craig T, Kaplan RM, Miller JE, Navarre C, et al. (2006) Anthelmintic resistance of gastrointestinal parasites in small ruminants. J Vet Intern Med20: 435-444.
- Lorimer SD, Perry NB, Foster LM, Burgess E, Douch PGC, et al.(1996) A Nematode Larval Motility Inhibition Assay for Screening Plant Extracts and Natural Products. J Agric Food Chem44: 2842-2845.
- Stepek G, Behnke JM, Buttle DJ, Duce LR(2004) Natural plant cysteine proteinases as anthelmintics. Trends Parasitol 20: 322-327.
- Shaik SA, Terrill TH, Miller JE, Kouakou B, Kannan G, et al.(2006)Sericea lespedezahay as a natural deworming agent against gastrointestinal nematode infection in goats. Vet Parasitol 139: 150-157.
- Kamaraj C, Rahuman AA, Elango G, Bagavan A, Zahir AA(2011) Anthelmintic activity of botanical extracts against sheep gastrointestinal nematodes, Haemonchuscontortus.Haemonchuscontortus.Parasitol Res 109: 37-45.
- Bidkar A, Ghadiali M, Patel C, ManojAswar M, Sanghai D, et al.(2012) Anthelmintc activities of the crude extracts of Allium cepa bulbs and Elletatriacardomomum Res J PharmaceutBiol and ChemSci 1: 50-58.
- Kaemmerer K,Butenkotter S(1973) The problem of residues in meat of edible domestic animals after application or intake of organophosphate esters. Residue Review 46: 1.
- Tariq kA, Chishti MZ, Ahmad F, Shawl AS(2009) Anthelmintic activity of extracts of Artemisia absinthium against Ovine nematodes. Vet Parasitol 160: 83?88.
- Hammond JA, Fielding D, Bishop SC(1997) Prospects for plant anthelmintics in tropical veterinary medicine. Vet Res Comm 21: 213-228.
- Yeap SK, Ho WY, Beh BK, Liang WS, Ky H, et al. (2010)Vernoniaamygdalina, an ethnoveterinary and ethnomedical used green vegetable with multiple bioactivities. J Med Plant Res 4: 2787-2812.
- Hennessy DR(1997) Physiology, pharmacology and parasitology. Int J Parasitol27: 145-152.
- Kabasa JD, Opuda-Asibo J, TerMeulen U(2000) The effect of oral administration of polyethylene glycol on faecalhelminth egg counts in pregnant goats grazed on browse containing condensed tannins. Trop Anim Health and Prod 32: 73-86.
- Savioli L, Albonico M, Engels D, Montresor A(2004) Progress in the prevention and control of schistosomiasis and soil-transmitted helminthiasis. ParasitolInt 53: 103-113.
- Hotez PJ, Molyneux DH, FenwickA, Kumaresan J, Ehrlich Sachs S,et al.(2007) Control of neglected tropical diseases. N Engl J Med. 357: 1027.
- Prichard RK, Hall CA, Kelly JD, Martin ICA, Donald AD(1980) The problem of anthelmintic resistance in nematodes. Aust Vet J 56: 239-251.
- Refaat J, Kamel MS, Ramadan MA, ALI AA(2012) Crinum; An endless source of bioactive principles: A review. Part 1- Crinum alkaloids: Lycorine-type alkaloids. International J Pharm Sci Res. 3: 1883-1890.
- Simelane MBC, Lawal OA, Djarova TG, Opoku AR(2010) In vitro antioxidant and cytotoxic activity of Gunneraperpensa(Gunneraceae) from South Africa. J Med Plant Res 4: 2181-2188.
- Martin JE (1953) British Veterinary Codex. Pharmaceutical Press, London, UK.
- http://www.stuartxchange.org/Tabako.html
- Grime AS, Nirmal SA, Bhalke RD, Chavan MJ(2008) Analgesic, CNS Depressant and Anthelmintic Activity of Sarcostemmaviminale. Iranian Journal of Pharmacology and Therapeutics 7: 153.
- Haman JC(2007) MateriaMedica in Veterinary herbal medicine. 24: 457-672.
- Singh R, Mehta A, Mehta S(2011) Anthelmintic activity of rhizome extracts of Curcuma longaand Zingiberofficinale (Zingiberaceae). International Journal of Pharmacy and Pharmaceutical Sci 3: 236-237.
- Van Wyk B-E, Wink M (2004) Medicinal plants of the world. Briza publications, Pretoria, South Africa.
- Olivier DK(2012) Theethnobotany and chemistry of South African traditional tonic plants. University of Johannesburg, Johannesburg, South Africa.
- McGaw LJ, Jäger AK, van Staden J(2000) Antibacterial, anthelmintic and anti-amoebic activity in South African medicinal plants. J Ethnopharmacol 72: 247-263.
- Ahmed M, Laing MD, Nsahlai IV (2013) In vitro activity of crude extracts of selected medicinal plants against Haemonchus contortus from sheep. J Helminthol 87: 174-179.
- Watt JM, Breyer-Brandwijk MG (1962) The medicinal and poisonous plants of southern and eastern Africa: being an account of their medicinal and other uses, chemical composition, pharmacological effects and toxicology in man and animal. Churchill Livingstone, London, UK.
- Maphosa V, Masika PJ (2010) Ethnoveterinary uses of medicinal plants: A survey of plants used in the ethnoveterinary control of gastrointestinal parasites of goats in the Eastern Cape, South Africa. Pharm Biol 48: 697-702.
- Vieira LS, Cavalcante ACR, Pereira MF, Dantas LB, Ximenes LJF (1999) Evaluation of anthelmintic efficacy of plants available in Ceara state, North-east Brazil, for the control of goat gastrointestinal nematodes. Rev Med Vet 150: 447-452.
- Marwat SK, Fazal-Ur-Rehman, Khan MA, Ahmad M, Zafar M, et al. (2011) Medicinal Folk recipes used as traditional phytotherapies in district Dera Ismail Khan, KPK, Pakistan. Pak J Bot 43: 1453-1462.
- Stepek G, Buttle DJ, Duce IR, Low A, Behnke JM (2005) Assessment of the anthelmintic effect of natural plant cysteine proteinases against the gastrointestinal nematode, Heligmosomoides polygyrus, in vitro. Parasitol 130: 203-211.
- Graham K, Graham EA, Towers GHN (1980) Cercaricidal activity of phenyl heptatriyne and α-terthienyl naturally occurring compounds in species of Asteraceae Compositae. Canadian J Zool 58: 1955-1958.
- Hoffman B, Hoelzl J (1988) New chalcones from Bidens pilosa. Planta Med 54: 52-54.
- Adongo OS (2013) Medicinal plants of Chuka community in Tharaka Nithi County, Kenya and some of their selected essential elements. Master thesis in applied and analytical chemistry, Kenyatta University.
- Rampadarath S, Puchooa D, Ranghoo-Sanmukhiya (2014) A comparison of polyphenolic content, antioxidant activity and insecticidal properties of Jatropha species and wild Ricinus communis L. found in Mauritius. Asian Pac J Trop Med 7: 384-390.
- Wafa G, Amadou D, Larbi KM, Héla EFO (2014) Larvicidal activity, phytochemical composition, and antioxidant properties of different parts of five populations of Ricinus communis L. Ind Crops Prod 56: 43-51.
- Harborne JB (1973) Phytochemical methods. Chapman Hall, London, p. 278.
- Boham BA, Kocipal-Abyazan R (1974) Flavonoids and Condensed Tannins from Leaves of Hawaiian Vaccinium reticulatum and V calycinum (Ericaceae). Pacific Science 48: 458-463.
- Porter LJ, Hrstich LN, Chan BG (1986) The conversion of procyanidins and prodelphinidins to cyanidins and delphinidins. Phytochemistry 25: 223-230.
- Makkar HPS (1995) Quantification of Tannins in Tree Foliage: A laboratory manual for the FAO/IAEA Co-ordinated Research Project on ‘Use of Nuclear and Related Techniques to Develop Simple Tannin Assays for Predicting and Improving the Safety and Efficiency of Feeding Ruminants on Tanniniferous Tree Foliage. Aleppo, Syria.
- Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18: 265-267.
- Webb JL (1963) Enzyme and Metabolic inhibitors. Academic Press, New York, USA.
- SAS (2000) Statistical Analysis System user guide (Version 8). SAS Institute Inc., SAS Campus Drive, Cary, N. C., USA.
- Lanusse C, Alvarez L, Lifschitz A (2014) Pharmacological knowledge and sustainable anthelmintic therapy in ruminants. Vet Parasitol 204: 18-33.
- Ademola IO, Akanbi AI, Idowu SO (2005) Comparative nematicidal activity of chromatographic fractions of Leucaena leucocephala seed against gastrointestinal sheep nematodes. Pharm Biol 43: 599-604.
- Hoste H, Jackson F, Athanasiadou S, Thamsborg SM, Hoskin SO (2006) The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends Parasitol 22: 253-261.
- Athanasiadou S, Kyriazakis I, Jackson F, Coop RL (2000) Consequences of long term feeding with condensed tannins on sheep parasitized with Trichostrongylus colubriformis. Int J Parasitol 30: 1025-1033.
- Molan AL, Waghorn GC, Min BM, McNabb WC (2000) The effect of condensed tannins from seven herbages on Trichostrongylus colubriformis larval migration in Vitro. Folia Parasitol 47: 39-44.
- Min BR, Hart SP (2003) Tannins for suppression of internal parasites. J Anim Sci 81: 102-109.
- Perry LM, Metzger J (1980) Medicinal plants of East and Southeast Asia: attributed properties and uses. MIT Press, Cambridge, London, UK.
- Duke JA (2000) Handbook of phytochemical constituents of GRAS herbs and other economic plants. CRC Press, Boca Raton, Florida, USA.
- Barrau E, Fabre N, Fouraste I, Hoste H (2005) Effect of bioactive compounds from Sainfoin (Onobrychis viciifolia Scop.) on the in vitro larval migration of Haemonchus contortus: role of tannins and flavonol glycosides. Parasitology 131: 531-538.
- Kerboeuf D, Riou M, Guegnard F (2008) Flavonoids and related compounds in parasitic disease control. Mini Review Med Chem 8: 116-128.
- Eguale T, Giday M (2009) In vitro anthelmintic activity of three medicinal plants against Haemonchus contortus. Int J Green Pharm 3: 29-34.
- Azando EVB, Hounzangbé-Adoté MS, Olounlade PA, Brunet S, Fabre N, et al. (2011) Involvement of tannins and flavonoids in the in vitro effects of Newbouldia laevis and Zanthoxylum zanthoxyloïdes extracts on the exsheathment of third-stage infective larvae of gastrointestinal nematodes. Vet Parasitol 180: 292-297.
- Nair JJ, Machocho AK, Campbell WE, Brun R, Viladomat F, et al. (2000) Alkaloids from Crinum macowanii. Phytochemistry 54: 945-950.
- Jin Z (2013) Amaryllidaceae and Sceletium alkaloids. Nat Prod Rep 20: 849-868.
- Madalaa NE, Piater L, Duberya I, Steenkamp P (2016) Distribution patterns of flavonoids from three Momordica species by ultra-high performance liquid chromatography quadrupole time of flight mass spectrometry: a metabolomic profiling approach. Revista Brasileira de Farmacognosia 26: 507–513.
- Makkar HPS, Francis G, Becker K (2007) Bioactivity of phytochemicals in some lesser-known plants and their effects and potential applications in livestock and aquaculture production systems. The Animal Consortium 1: 1371-1391.
- Rochfort S, Parker AJ, Dunshea FR (2008) Plant bioactives for ruminant health and productivity. Phytochemistry 69: 299-322.
- Alonso-Díaz MA, Torres-Acosta JFJ, Sandoval-Castro CA, Hoste H, Aguilar-Caballero AJ, et al. (2009) Sheep preference for different tanniniferous tree fodders and its relationship with in vitro gas production and digestibility.. Anim Feed Sci Technol. 151: 75-85.
- Kubola J, Siriamornpun S (2008) Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia) leaf, stem and fruit fraction extracts in vitro. Food Chem 110: 881-890.
- Butts CT, Zhang X, Kelly JE, Roskamp KW, Unhelkar MH, et al. (2016) Sequence comparison, molecular modeling, and network analysis predict structural diversity in cysteine proteases from the Cape sundew, Drosera capensis. Comput Struct Biotechnol J 14: 271-282.
- Kundu S, Roy S, Lyndem LM (2014) Broad spectrum anthelmintic potential of Cassia plants. Asian Pac J Trop Biomed 4: 436-441.
- Barry TN (1985) The role of condensed tannins in the nutritional value of Lotus pedunculatus for sheep. Brit J Nutr 54: 211-217.
- Quijada J, Fryganas C, Ropiak HM, Ramsay A, Mueller-Harvey I, et al. (2015) Anthelmintic activity against Haemonchus contortus or Trichostrongylus colubriformis from small ruminants are influenced by structural features of condensed tannins. J Agric Food Chem 63: 6246-6354.
- Adamu H, Endeshaw T, Teka T, Kife A, Petros, B (2006) The prevalence of intestinal parasites in paediatric diarrhoeal and non-diarrhoeal patients in Addis Ababa Hospitals, with special emphasis on opportunistic parasitic infections and with insight into the demographic and socio-economic factors. Ethiop J Health Dev 20: 39-46.
- Villalba JJ, Provenza FD (2009) Learning and Dietary Choice in Herbivores. Rangeland Ecology and Management 62: 399-406.
- Solaiman SG (2010) Goat Science and Production. John Wiley & Sons, Hoboken, New Jersey, USA.
- Werne S, Perler E, Maurer V, Probst JK, Hoste H, et al. (2013) Effect of sainfoin (Onobrychis viciifolia) and faba bean (Vicia faba) on the periparturient rise in ewes infected with gastrointestinal nematodes. Small Ruminant Res 113: 454-460.
- Desrues O, Mueller-Harvey I, Pellikaan WF, Enemark HL, Thamsborg SM (2017) Condensed tannins in the gastrointestinal tract of cattle after Sainfoin (Onobrychis viciifolia) intake and their possible relationship with anthelmintic effects. J Agric Food Chem 65: 1420-1427.
- Mottier MD, Prichard RK (2008) Genetic analysis of a relationship between macrocyclic lactone and benzimidazole anthelmintic selection on Haemonchus contortus. Pharmacogenet Genomics 18: 129-140.
Citation: Fomum SW, Nsahlai IV (2019) In Vitro Evaluation of Anthelmintic Interaction of Plant Species Combinations Putatively Containing Similar Bioactive Macromolecules in Sheep. J Altern Complement Integr Med 5: 071.
Copyright: © 2019 Sylvester W Fomum, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

