Pathological Findings in Neck Dissections of Head and Neck Cancer, and their Associations with outcome
*Corresponding Author(s):
Shiner YDepartment Of Otolaryngology Head And Neck Surgery, Carmel Medical Center, Haifa, Israel
Tel:04-8250279,
Email:yotamsh@clalit.org.il
Abstract
Importance: Consensus has not been reached regarding the prognostic value of various pathological factors, including extranodal extension (ENE), in patients with head and neck squamous cell carcinoma (HNSCC).
Objective: To evaluate associations of pathological findings in neck dissection on survival rates of HNSCC
Design: A retrospective study of patients with HNSCC treated during 2009-2017, either surgically (Surgery Group) or with radiotherapy or chemotherapy, followed by neck dissection (Organ Preservation Group). The respective mean follow-up periods were 50.1±44.1 and 43.3±27.4 months.
Setting: A tertiary care university-affiliated medical
Patients: 135 patients were selected consecutively. After excluding 36 with oropharyngeal cancer, nasopharyngeal carcinoma, or an unknown primary, the cohort comprised 72 patients in the Surgery Group and 27 in the Organ Preservation
Exposure: Surgical treatment or the combination of radiotherapy/chemoradiotherapy and neck dissection.
Main outcomes: Five-year overall survival and associations of demographic and nodal Characteristics with
Results: The Surgery Group comprised 53/72 males, mean age 71.6±12.96 years. The Organ Preservation Group comprised 26/27 males, mean age 71.3±10.5 years. For the respective groups, the mean values of nodal yield were 30.54±13.09 and 18±9.33, and the mean numbers of pathological lymph nodes were 2.09±3.7 and 0.88 ± 1.8; ENE was detected in 39% and 22%, respectively. In the Surgery Group, the AJCC 8th edition guidelines upstaged 28/53 patients (53%) with nodal metastases. The 5-year overall survival rate was 44.7%: 31% vs 55% for patients with without ENE (p=0.037). In a multivariate Cox proportional-hazard model, age and the number of pathological nodes were associated with overall survival. In the Organ Preservation Group, the 5-year overall survival rate was 44%. In a multivariate Cox model, ENE and the number of pathological nodes were associated with overall survival. None of the patients with ENE in the last group survived at 5 years.
Conclusion: Among patients with HNSCC, the number of positive nodes in neck dissection was associated with overall survival, both in those for whom surgery was first-line treatment and those who underwent surgery following chemoradiotherapy. However, ENE was associated with overall survival in the latter group only, with fatal outcome.
Background
For several decades the status of metastatic cervical lymph nodes has been known to impact the prognosis of patients with head and neck malignancy. Expected survival drops by half when a patient presents with one metastatic node [1]. A major change of the 8th edition of the American Joint Committee for Cancer Tumor-Node- Metastasis (AJCC TNM) is the incorporation of extra nodal extension (ENE) in nodal staging. ENE is an adverse prognostic factor that changes the characteristics of the entire nodal stage [2]. The AJCC 8th edition upstages a single metastatic lymph node with ENE of up to 3 cm, from N1 to N2a; whereas all other advanced nodal metastases (N2a, N2b, N2c and N3) with ENE are upstaged to N3b, regardless of their size or location. Recent studies suggest poor prognostic utility of the current AJCC edition compared with the previous one [3]. The impact of some pathological findings in neck dissection is well established. Yet, consensus has not been reached regarding the contribution of the various factors to prognosis. To emphasize this point, some authors reported that ENE is a pathological finding with a very high prognostic value, whereas others consider it a common finding that does not contribute to predicting a patient’s prognosis and survival [4].
More recently, the number of metastatic nodes was found to be a significant predictor of overall survival in HNSCC [5], and oral cavity carcinoma [6]. The impact of these pathological findings in patients who underwent planned or salvage neck dissection following chemo-radiotherapy is not clear. Only a few authors have described pathological findings in neck dissections following chemo- radiation, including the quantity and size of pathological nodes that may indicate worse prognosis [7,8]. Furthermore, adjuvant treatments are generally not recommended for patients with adverse features in neck dissection following chemo-radiation.
The current study examined pathological findings in neck dissection in patients with HNSCC who underwent either surgical treatment as primary treatment or neck dissection following organ preservation treatment. We assessed the associations of these findings with staging in the former group and outcomes in both groups.
Methods
This is a retrospective study of patients with HNSCC who were treated surgically (Surgery Group) and patients who were treated with organ preservation (radiotherapy or chemo radiotherapy) followed by either planned or salvaged neck dissection (Organ Preservation Group) during 2009-2017 in the Department of Otolaryngology, Head and Neck Surgery, Carmel Medical Center. Study inclusion criteria were: HNSCC, whose primary treatment was either surgery including neck dissection, or organ preservation that was followed by neck dissection; and a minimum follow-up period of 12 months. Prior treatment for HNSCC and a prior history of head and neck malignancy were exclusion criteria. In addition, patients were excluded from the analysis who had SCC of the oropharynx, of the nasopharynx or of an unknown primary tumor. This is due to the different staging for nodal metastasis for these primary sites and the lack of HPV status in these patients, which was incorporated in the new AJCC staging. Patients who had undergone neck dissection for non-head and neck or non-SCC primaries were also excluded.
For every patient included in the analysis, the following data were accessed: demographic details, clinical details including the presentation and staging of disease, treatment modalities, follow-up data and outcomes. During neck dissection, neck levels were marked by the surgeon, and the specimens were submitted to pathology divided into neck levels. The pathological parameters of the tumor included: the total number of nodes and the number of positive nodes in the pathologic specimen, the size of the greatest node, evidence of ENE, and anatomic location and spread. The disease was initially staged according to the valid AJCC staging method and re-staged according to the AJCC 8th edition. All the above-mentioned parameters were verified using the relevant Computerized Medical Records in use in our institute.
Statistical Analysis
All the parameters examined were analyzed for normal distribution. Correlations between variables were done using the Pearson`s and the Spearman`s coefficients of correlation, for parametric and non-parametric groups, respectively. Univariate analyses of survival and cause specific survival for the measured variables were performed by constructing Kaplan Meier curves. Statistical significance between subgroups was tested using the Log-Rank test. Cox's proportional hazard model was used to evaluate associations of continuous variables (age, number of positive nodes, size of the greatest node) with survival. To evaluate the intervariable relationships and to detect independent predictors of survival, the multivariate Cox`s proportional hazard model was performed. For all analyses, P<0.05 was considered significant.
Results
A total of 135 patients were identified from the medical records; of them, 36 were excluded from the analysis due to the presence of oropharyngeal carcinoma, nasopharyngeal carcinoma or carcinoma of an unknown primary. Hence, 99 patients who had undergone 139 neck dissections were included in the analysis.
Patients with surgical treatment (Surgery Group):
The surgery group comprised 72 patients, who underwent 95 neck dissections. Of them, 23 had a bilateral neck dissection; 53 patients were males (73.6%). The mean age was 71.6±12.96 years and the mean follow-up was 50.1±44.1 months. The most common primary site in this group was cutaneous (33 patients, 46%); 21 patients (29%) had laryngeal cancer and 18 (25%) oral cavity cancer. The mean nodal yield was 30.54±13.09 in the ipsilateral side and 17.17±18.90 in the contralateral side.
Patients with neck dissection following chemo-radiotherapy (Organ Preservation Group):
The Organ Preservation Group comprised 27 patients who underwent 44 neck dissections; 17 patients had undergone bilateral neck dissection. This group included 26 males and one female. The mean age was 71.3±10.5 years and the mean follow-up 43.3±27.4 months. The majority of patients had laryngeal carcinoma (23 patients, 85%), whereas 3 (11%) had cutaneous SCC and one (4%) had an oral cavity carcinoma. The mean nodal yield in this group was 18±9.33 in the ipsilateral side and 14.76±11.60 nodes in the contralateral side.
Pathological lymph nodes:
For the Surgery Group and the Organ Preservation Groups, the mean numbers of pathological lymph nodes were 2.09±3.7 and 0.88 ± 1.8, respectively. The respective mean sizes of the greatest node were 1.75 ± 1.5 cm and 0.75 ± 1.2 cm. ENE was detected in 39% of the surgery group (28 of 72 patients), and in 22% of the Organ Preservation Group (6 of 27 patients).
N stage of the surgical group:
(Table 1) compares N staging according to the 7th AJCC and the 8th AJCC systems, for patients with head and neck squamous cell carcinoma who were treated surgically.
|
Primary Origin |
7th AJCC TNM () |
8th AJCC TNM |
|
N0 |
19(26%) |
19(26%) |
|
N1 |
23(31%) |
13(18%) |
|
N2a |
7(10%) |
12(17%) |
|
N2b |
21(29%) |
10(14%) |
|
N2c |
3(4%) |
0 |
|
N3a |
0 |
0 |
|
N3b |
0 |
18(25%) |
Table 1: N staging according to the 7th AJCC and the 8th AJCC systems, for patients with head and neck squamous cell carcinoma who were treated surgically.
After re-staging the surgery group according to the 8th AJCC staging criteria, changes in N staging were as follows: N0- no change; N1- 10/23 patients (41%) were upgraded to N2a; N2a: 4/6 patients (66%) were upgraded to N3b; N2b:11/21 (52%) were upgraded to N3b. The 3 patients (100%) with N2c were upgraded to N3b.
Outcomes and impacts of pathological findings: Surgery Group
The prevalence of ENE for this group differed according to the sites of the primary tumor. In patients with laryngeal primary tumors, the rate of ENE was 19% (4/21 patients), similar to the rate of ENE in oral cavity primary, 22% (4/18 patients). These rates were significantly (p=0.002) lower than the rate of ENE among patients with cutaneous primary tumors, 66% (20/33 patients). After exclusion of the N0 patients, ENE presented in 44% of laryngeal tumor neck metastases (4/9 patients) and in 33% of oral cavity tumors with clinical neck metastasis (4/13), compared to 65% of primary cutaneous tumors (20/31 patients).
Regarding the Surgery Group, 5-year overall survival was 44.7%. It was significantly lower for patients with ENE than for patients without ENE (31% vs. 55%, p=0.037) (Figure 1). Overall, 28 of 72 (39%) patients in the surgery group were upstaged, compared to the previous N staging. Five-year overall survival according to the new nodal staging was as follows: N0-58%, N1-59%, N2a-40%, N2b-40% and N3b-21% (p=0.0034) (Figure 2). Further, in a univariate Cox proportional hazard model, the following parameters were found to be associated with overall survival (Table 2): the size of the greatest node (p=0.0115), the number of positive nodes
(p=0.008) and patients’ age (p=0.0014). In a multivariate Cox proportional hazard model, the parameters that were most strongly associated with overall survival were patients’ age (p=0.0062), followed by the number of pathological nodes (p=0.046). The parameters: ENE, the new 8th AJCC nodal staging and the size of the greatest node became insignificant.
|
Prognastic factor for overall survival of patients in the surgery group |
First Model |
Second Model |
Third Model |
|||
|
|
X2 Value |
P value |
X2 Value |
P value |
X2 Value |
P value |
|
Age of patient |
7.61 |
0.0058 |
7.61 |
0.0058 |
7.48 |
0.0062 |
|
Numbr od positive nodes |
3.4 |
0.0651 |
3.4 |
0.0468 |
3.9 |
0.0464 |
|
Size of greatest node |
2.1 |
0.146 |
2.2 |
0.134 |
3.4 |
0.064 |
|
Presence of ENE |
0.005 |
0.942 |
Removed |
|
||
|
New N Staging |
1.07 |
0.898 |
1.06 |
0.899 |
Removed |
|
Table 2: Multiple stages of three multivariate Cox proportional hazard models for overall survival of patients with head and neck squamous cell cancer who were treated by surgery.
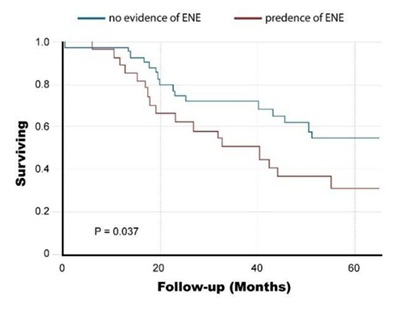 Figure 1: Survival Rates of the Surgery Group according to the presence of extra nodal extension
Figure 1: Survival Rates of the Surgery Group according to the presence of extra nodal extension
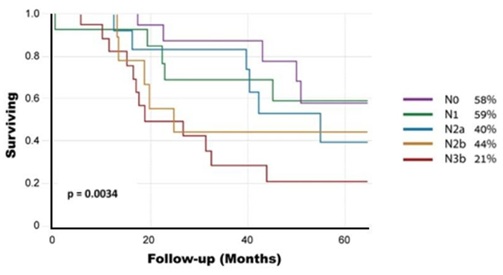 Figure 2: Survival Rates of the Surgery Group according to the new N staging
Figure 2: Survival Rates of the Surgery Group according to the new N staging
Outcomes and associations of pathological findings of neck dissection following organ preservation
In the Organ Preservation Group, 5-year overall survival was 44.1%. However, the presence of ENE in neck dissection specimens was fatal. None of the patients who had ENE in neck dissection following organ preservation treatment survived 5 years. Overall, 5-year survival (Kaplan Meier) was 0% for patients with ENE compared to 54% for patients without ENE (p=0.0008) (Figure 3). Cox's proportional hazards model identified the following parameters as associated with 5-year overall survival: ENE (P=0.0005), the number of pathological nodes (p=0.035) and the size of the greatest node (p=0.006). In a multivariate Cox's model, ENE and the number of pathological nodes were found to be significant. (P=0.005 and P=0.036, respectively).
 Figure 3: Survival rates of the Organ Preservation Group according to the presence of extranodal extension
Figure 3: Survival rates of the Organ Preservation Group according to the presence of extranodal extension
Discussion
The current study demonstrated a significant proportion of nodal upstaging in patients with HNSCC, according to the AJCC 8th edition, due to the inclusion of ENE in the guidelines. The greatest changes were found in patients with cutaneous SCC. A higher proportion of upstaging due to the presence of ENE was reported by Moeckelman et al., who found up to 75% upstaging of the study population, which was composed of cutaneous and oral cavity primaries [9]. Another study, by Liu et al, showed that inclusion of ENE resulted in upstaging by up to 50% of the study population10. The same group examined patients with a primary cutaneous tumor and reported upstaging of 75% of this patient group.
In our study, 60% of patients with cutaneous SCC were upstaged, compared to 28% and 19% of those with oral cavity and larynx primary, respectively. A possible explanation lies in differences in the period lapsed between the diagnosis of the primary disease and the surgical intervention in the neck. In laryngeal and oral cavity primary SCC tumors, neck dissection is performed as part of the mandatory initial treatment and is combined with surgical treatment in the primary site, within a short time after diagnosis. In contrast, patients with cutaneous tumors undergo resection of the primary tumor, mostly by plastic surgeons, and are referred for further treatment by head and neck surgeons following the clinical appearance of neck metastasis, months or even years following the diagnosis of the primary skin SCC. Hence, the prolonged incubation time of metastases in cervical lymph nodes might explain the ENE. The time factor has crucial importance in the treatment of patients with cervical metastases.
The circumstances of the neck dissection are also important: Notably, most patients with primary tumors in the larynx and oral cavity underwent elective neck dissection, without clinical evidence of neck metastasis, due to a high risk of occult metastases. In contrast, patients with primary cutaneous tumors were operated following clinical diagnosis of neck metastases. Even after excluding patients with pathological N0, the rate of ENE remained higher in those with cutaneous primary disease. For the surgery group, ENE was associated with overall survival in univariate survival analysis but not in multivariate survival analysis. Most patients with pathological ENE in our department were treated with adjuvant chemo-radiotherapy since 2004. This may have affected the association of ENE with survival in this group, in our retrospective cohort.
Our study included patients with cancers of the oral cavity, larynx and cutaneous SCC primary. This might raise questions about the natural behavior of malignancies of different origins in the head and neck. However, regarding nodal staging, both in the previous and in the present staging system editions, the N stage of these origins are identical. Moeckelmann et al. suggested that while the nodal staging system provides some prognostic information for patients with metastatic oral cavity origin, it does not provide any risk stratification for patients with metastatic cutaneous origin [9]. Furthermore, in that study, the population of patients with cutaneous origin was older than of the other groups. Accordingly, the most significant adverse independent risk factor in our study was patients’ age. Notably, for cutaneous primary SCC, the index primary is often unknown or of limited prognostic relevance. Hence, N staging is frequently used in isolation, as a surrogate for disease stage.
Some authors have argued that ENE is a common finding in metastatic HNSCC that lacks good prognostic ability [10]. ENE was present in 40% of our study population, and in even higher rates, up to 80%, in other large cohorts. Some authors also claim that despite its being an important adverse prognostic factor, ENE has failed to serve as an adequate prognostic discriminator in metastatic cutaneous SCC. In the surgery group, ENE was found to be a significant predictor for overall survival in the univariate model. Yet, in multivariate analysis, it was not significant. In contrast, the number of positive nodes was found to be the most significant independent predictor for overall survival, rather than ENE or the new AJCC N staging. Son et al. showed similar results, in which the number of positive lymph nodes rather than the presence of ENE, was a prognostic factor [11]. The number of positive lymph nodes also showed strong prognostic value in other reports. From their analysis of the National Cancer Database, Divi et al. suggested that a positive lymph node yield from neck dissection can predict mortality in head and neck cancer [12].
In a study that included 14,554 patients with oral cavity SCC, Ho et al. argued that mortality risk escalated continuously as the number of metastatic nodes increased, without reaching a plateau. The most pronounced effect was found at four metastatic lymph nodes. Moreover, the number of metastatic nodes was found to be a critical predictor of oral cavity SCC mortality, eclipsing the prognostic value of other features such as the size of nodes and contra laterality [6]. Liao et al. suggested a subdivision based on the prognostic value of positive nodes and ENE. Accordingly, patients with more than 8 positive nodes or more than 5 ENE findings would have an unfavorable prognosis and be classified as pN3b. In contrast, patients with positive nodes, less than 8 positive nodes or less than 5 ENE findings, would be classified as pN3a.
Another factor to be considered in evaluating overall survival is nodal yield. A recently published clinical review [13] suggested that increased nodal yield in neck dissection specimens of HNSCC, most commonly described as ≥18 lymph nodes, is associated with improved overall survival. They concluded that increased nodal yield might be used as a prognostic marker. Most published studies referred to oral cavity primary tumors, and in those studies, overall survival improved with a greater nodal yield. In contrast, among patients with laryngeal SCC, treated with neck dissection, increasing the nodal yield was not associated with an increased probability of a change in nodal stage [14]. In our study, the mean nodal yield was higher in the Surgery Group (40±21.62) than in the Organ Preservation Group (29.48±18.16). The meaning of this finding is not clear, since its prognostic significance is inconclusive. This is especially in regard to a dissection that followed prior radiotherapy or chemoradiotherapy, which could cause scarring and fibrosis of the lymphatic tissue and the neck planes.
Most of our patients who were upstaged due to the new classification were finally categorized as pN2a and pN3b. One of the most obvious trends was a shift of all three patients initially classified as stage pN2c, to pN3b. Other studies reported such shift in classification in 64-73% of patients [9,10,15]. An important finding of our cohort is that a new pathological staging category, namely pN3a, did not include any patients. The meaning is that none of our patients presented with a metastatic cervical lymph node larger than 6 cm without evidence of ENE. The same finding was described in other publications. The meaning is that patients with metastatic lymph nodes larger than 6cm are no longer candidates for surgical treatment and are all referred to chemoradiotherapy prior to surgical intervention. Despite the good prognostic effect on overall survival in univariate analysis, in multivariate analysis the new N staging and the presence of ENE were not statistically significant. These two parameters were found to interact with each other, and thus to become insignificant. However, even after exclusion of ENE from the analysis, the new N staging was still insignificant, in the presence of age and multiple positive nodes.
Contrasting with the above, the presence of ENE in neck dissection in the Organ Preservation Group of our study contributed to determining the prognostic outcome of patients. This is because none of the patients had reached 5-year survival. On multivariate analysis, ENE was an independent prognostic factor for overall survival after salvage surgery. Patients who were treated with chemo-radiotherapy, and found to have pathological nodes with ENE, most probably had highly resistant disease, which affected their outcome. Our study has some limitations due to the retrospective design and the relatively small cohort. Further, several sites of head and neck cancer were included.
In conclusion, according to our study, pathologic findings such as ENE, and the size and number of positive metastatic nodes in ND, are good prognostic factors for overall survival among patients whose first treatment was surgical neck dissection. However, in multivariate analysis, age and the number of positive nodes were found to be most strongly associated with overall survival. In the Organ Preservation Group, both a higher number of pathological nodes and ENE were found to be associated with poor prognosis. The presence of ENE among these patients was shown to be fatal.
References
- Cerezo L, Millan I, Torre A, Aragon G, Otero J, et al. (1992) Prognostic factors for survival and tumor control in cervical lymph node metastases from head and neck cancer. A multivariate study of 492 cases. Cancer 69: 1224-1234.
- Pollaers K, Hinton-Bayre A, Friedland PL, Farah CS (2018) AJCC 8th Edition oral cavity squamous cell carcinoma staging - Is it an improvement on the AJCC 7th Edition? Oral Oncol 82: 23-28.
- Lydiatt WM, Patel SG, Sullivan BO, Brandwein MS, Ridge JA, et al. (2017) Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer JClin 67: 122-137.
- Moeckelmann N, Ebrahimi A, Dirven R, Liu J, Low T-HH, et al. (2018) Analysis and comparison of the 8th edition american joint committee on cancer (AJCC) nodal staging system in cutaneous and oral squamous cell cancer of the head and neck. Ann Surg Oncol 25: 1730-1736.
- Roberts TJ, Colevas AD, Hara W, Holsinger FC, Oakley-Girvan I, et al. (2016) Number of positive nodes is superior to the lymph node ratio and American Joint Committee on Cancer N staging for the prognosis of surgically treated head and neck squamous cell carcinomas. Cancer 122: 1388-1397.
- Ho AS, Kim S, Tighiouart M, Gudino C, Mita A, et al. (2016) Metastatic lymph node burden and survival in oral cavity cancer. J Clin Oncol 35: 3601-3609.
- Brizel DM, Prosnitz RG, Hunter S, Fisher SR, Clough RL, et al. (2004) Necessity for adjuvant neck dissection in setting of concurrent chemoradiation for advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 58: 1418-1423.
- Rengan R, Pfister DG, Lee NY, Kraus DH, Shah JP, et al. (2008) Long-term neck control rates after complete response to chemoradiation in patients with advanced head and neck cancer. Am J Clin Oncol 31: 465-469.
- Moeckelmann N, Ebrahimi A, Tou YK, Gupta R, Low T-HH, et al. (2018) Prognostic implications of the 8th edition American Joint Committee on Cancer (AJCC) staging system in oral cavity squamous cell carcinoma. Oral Oncol 85: 82-86.
- Liu J, Ebrahimi A, Low T-HH, Gao K, Palme CE, et al. (2018) Predictive value of the 8th edition American Joint Commission Cancer (AJCC) nodal staging system for patients with cutaneous squamous cell carcinoma of the head and neck. J Surg Oncol 117: 765-772.
- Son HJ, Roh JL, Cho KJ, Choi SH, Nam SY, et al. (2018) Nodal factors predictive of recurrence and survival in patients with oral cavity squamous cell carcinoma. Clin Otolaryngol 43: 470-476.
- Divi V, Chen MM, Nussenbaum B, Rhoads KF, Sirjani DB, et al. (2016) Lymph node count from neck dissection predicts mortality in head and neck cancer. J Clin Oncol 34: 3892-3897.
- De Kort WWB, Maas SLN, Van Es RJJ, Willems SM (2019) Prognostic value of the nodal yield in head and neck squamous cell carcinoma: A systematic review. Head Neck 41: 2801-2810.
- Bottcher A, Dommerich S, Sander S, Rhoads KF, Sirjani DB, et al. (2016) Nodal yield of neck dissections and influence on outcome in laryngectomized patients. Eur Arch Otorhinolaryngol 273: 3321-3329.
- Canueto J, Burguillo J, Moyano-Bueno D, Vinolas-Cuadros A, Conde-Ferreiroset A, et al. (2019) Comparing the eighth and the seventh editions of the American Joint Committee on Cancer staging system and the Brigham and Women’s Hospital alternative staging system for cutaneous squamous cell carcinoma: Implications for clinical practice. J Am Acad Dermatol 80: 106-113.
Citation: Shiner Y, Copty A, Doweck I (2021) Pathological Findings in Neck Dissections of Head and Neck Cancer, and their Associations with outcome. J Otolaryng Head Neck Surg 7: 57
Copyright: © 2021 Shiner Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.