
Persistence and Recurrence of Ovarian Endometrioma
*Corresponding Author(s):
Salgado IMADepartment Of Gynecology And Obstetrics, State Public Servants Hospital - Francisco Morato De Oliveira, São Paulo, Brazil
Tel:+55 21983320824,
Email:bella.imas@gmail.com
Abstract
Porpouse: Analyze the incidence of persistence and recurrence of ovarian endometriomas in patients at a reference center and evaluate possible associated factors.
Methods: A cohort study with patients histologically diagnosed with ovarian endometriomas, attended at a reference center for endometriosis and pelvic chronic pain at State Public Servants Hospital - Francisco Morato de Oliveira, from 01/01/2003 to 01/07/2019. Follow up was registered in clinical charts, from which we analyzed epidemiological data, characteristics of the disease, type of surgery, medical treatment, its interruption and follow up time. A logistic regression model was used to evaluate predictors associated with persistence and recurrence, separately.
Results: We analysed data from 293 patients, with a median follow up time of 74.16 months (40.26 - 112.73). Persistence was found in 9.87% (37) of patients after surgery and overall rate of recurrence was 20.27% (77). “Drainage of endometriomas” and “GnRH agonist use” were both correlated to persistence, OR: 9.09 (3.29-25.12) and OR: 7.16 (2.53-20.25), respectively. The only predictor associated with recurrence was “interruption of clinical treatment”, with OR: 5.93 (2.81-12.53) adjusted by infertility, cyst size (cm), bilaterality of cystic lesions and surgical technique of drainage.
Conclusion: Ovarian endometriomas had a persistence rate of 9.87% and recurrence rate of 20.27%. Drainage of endometriotic cyst and its complementary treatment with GnRH agonist were associated with greater chance of persistence. Postoperative medical treatment’s interruption was independently associated with greater chance of recurrence and inversely to time elapsed of the recurrence.
Keywords
Endometrioma; Endometriosis; Persistence; Recurrence; Risk factors
INTRODUCTION
Endometriosis is characterized by the growth of endometrial tissue outside the uterine cavity, which induces a chronic inflammatory reaction [1]. These deposits undergo cyclic proliferation in response to hormones, mainly estrogen [2,3]. It affects 2-10% of women of reproductive age, and it may cause subfertility, dysmenorrhea, dyspareunia and chronic pelvic pain, leading to a serious impact on quality of life [1,3,4]. Although its pathogenesis is still unknown, there have been many hypotheses to explain the underlying pathophysiology of endometriosis, including the theory of retrograde menstruation, metaplasia of the mesothelium or peritoneum and implantation of viable endometrial cells [5,6]. Recent studies also show augmented cell viability in eutopic endometrium from these patients, as consequence of a reduction in cell death by apoptosis, which may facilitate their invasive character [6].
Macroscopically, three types of endometriotic lesions must be considered: superficial peritoneal, cystic ovarian and deep infiltrating [5]. Besides variable locations, theses lesions can present differences at color, size and extent, ranging from a few small superficial lesions on otherwise normal pelvic organs to extensive fibrosis and adhesion formation causing marked distortion of pelvic anatomy [4]. Clinically, ovarian endometriomas are the most common finding in patients with endometriosis, accounting for 17 to 44% of the cases [4,7]. They represent pseudocysts formed by menstrual blood deposition in ovarian cortex [8,9]. Its suspicion may be raised by clinical features and imaging studies. However, the gold standard for the diagnosis is laparoscopic inspection, which allows direct visualisation and biopsy of dark brown ovarian cysts [3,8].
Hormonal therapy, surgery or their combination are the main therapeutic options available [7]. Ovulation seems crucial in the pathogenesis of ovarian endometriomas [10]. Therefore, hormonal therapy inducing amenorrhea should increase the efficacy of treatment by down-regulating the estrogen level and reaching endometrium atrophy [2]. It is also standard treatment for pain and there is evidence that it can limit progression of lesions [11]. Surgical removal can be recommended when clinical treatment fails to control pain symptoms and laparoscopic surgery is considered the preferable approach. Several surgical techniques can be adopted to manage endometriomas, such as drainage, coagulation, laser vaporization or excision/cystectomy [9]. Less traumatic procedures could preserve ovarian function and avoid further thermal trauma caused by electrosurgical hemostasis of stromal vessels [9]. However, simple coagulation or vaporization would result in the persistence of ectopic endometrium and an increase in the risk of short-term cyst recurrence [4,9]. Therefore, as compared with drainage and ablation, excisional surgery provides more favorable outcomes with regard to recurrence of endometrioma, recurrence of symptoms, and subsequent spontaneous pregnancy in infertile women, although it may decrease ovarian reserve [9,12,13].
Recurrence risk increases if the lesions are not completely removed at surgical approach, and they tend to arise on the same location [14]. Also, even the most pristine surgical technique cannot guarantee removal of all microscopic lesions and therefore hormonal therapy is highly recommended after surgery [13,14]. The aim of postoperative medical treatment is suppressing ovarian activity and, in turn, leading to atrophy of the lesions [14]. There is also evidence that it reduces pain and improves quality of life of these patients [11]. After surgical removal, relapse of symptoms occurs in 40-45% of women [3]. Owing to today’s easy and reliable detection of ovarian endometriotic cysts by means of transvaginal ultrasonography, data are accumulating on factual postoperative endometrioma recurrence rates, which reportedly vary between 30 and 50% after two to five years of follow-up [10]. The term recurrence is therefore used for both persistent lesions at a short-time follow up and those arising later, after imaging studies showing no evidence of the disease.
Considering its effects on quality of life and its economic burden due to the necessity of costly medical and surgical treatments, the recurrence of endometrioma is one of the most important unresolved problems in the management of ovarian endometriosis [2,7]. Although the pathogenesis in not fully understood, there have been two hypotheses that seek to explain the underlying pathophysiology of endometrioma recurrence: growth from residual lesions, or the development of retrograde menstruation after surgery [1,15]. However, there is no consensus on its risk factors in the literature. The aim of this study is to estimate persistence and recurrence rates of ovarian endometriomas in patients followed at a reference center and evaluate possible associated factors.
METHODS
Our proposal is a cohort study with patients histologically diagnosed with ovarian endometriomas, attended at a reference center for endometriosis and chronic pelvic pain at State Public Servants Hospital - Francisco Morato de Oliveira, from 01/01/2003 to 01/07/2019. The Research Ethics Committee from the same institution approved the study (CAAE: 36271213.8.0000.5463). Women with surgical diagnosis of ovarian endometrioma, confirmed by histological findings, were eligible. Patients who were clinically diagnosed with menopause, previous or due to surgery, were excluded from analysis. Those with insufficient follow up time, established as less than 12 months, were also excluded, as well as those women with insufficient data from their surgical procedures.
All patients had specific clinical charts, applied at their first visit to the reference center after histological confirmation. Clinical data were prospectively recorded at each return, including characteristics of the disease, type of surgery, clinical treatment, its interruption and follow up time. Epidemiological data analyzed was: age, profession, ethnicity, Body Mass Index (BMI), educational level, parity, smoking, alcoholism, exercise practice, comorbidities, use of hormonal contraceptives and its interruption during follow up. Characteristics of the disease included: symptoms, size of cysts, serum CA 125 levels pre and postoperative and disease staging. Some demographic data were filled retrospectively through medical charts revision.
Persistence was stated as an ultrasonographic image compatible with endometrioma, larger than 2cm, at the first exam after surgery. Recurrence was defined as a persistent ultrasonographic image compatible with endometrioma, larger than 2cm, shown after a normal exam or at least 6 months after surgery. Data was export to a Windows Excel spreadsheet. Categorical variables were expressed as percentage. Continuous variables with normal distribution were presented as mean and Standard Deviation [SD]); non-normal variables were reported as median and Interquartile Range [IQR]). A logistic regression model was used to evaluate predictors associated with persistence of lesions or recurrence, separately. All statistical analyses were performed using a standard software package (Stata, version 12.0; StataCorp), with a significant confidence interval of 95%.
RESULTS
From 388 patients with proved ovarian endometriosis, 52 were excluded of statistical analysis due to insufficient follow up time after surgery. We also excluded 19 with lack of surgical data and 24 who underwent bilateral oophorectomy as surgical treatment, resulting in 293 patients eligible for epidemiological analysis and 375 ovarian endometriomas, as shown in flowchart 1. Persistence and recurrence were evaluated according to affected ovaries individually. Age ranged from 17 to 54 years old, with mean age of 38.68 (±6.35). Mean BMI was 26.04 (±5.29), with 53.24% of them (156) presenting overweight or obesity. Regular exercise activity was reported in 21.53% (62) of the charts, at two or more days of the week. All patients denied use of drugs and 99.21% (290) denied alcohol consumption. Comorbidities were found in 66.95% (162) of time, the most frequent: chronic hypertension (19.01%), uterine leiomyomas (7.84%) and psychiatric disorders (6.61%). Self-reported ethnicity revealed that 73.40% (207) were considered white, 24.47% (69) black and 2.48% (6) Asian women. Regarding educational level, 68.28% (173) completed Higher Education, 33.21% (88) Secondary Education and 1.51% (4) only Primary Education. Civil union was found in 63.89% (184) of women and 6.16% (18) of them never have had sexual experience. 90 of 138 (65.22%) women who have had given birth had at least one cesarean delivery. There were 93.69% (267) with regular menstrual cycles.
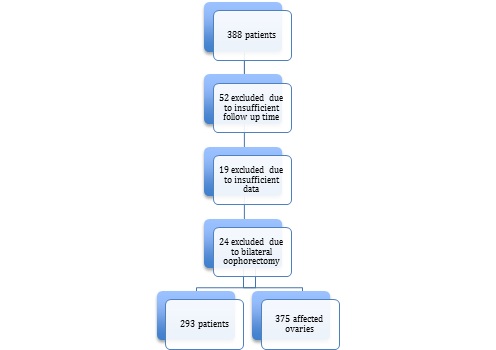 Flowchart 1: Patients’ eligibility for analysis.
Flowchart 1: Patients’ eligibility for analysis.
Symptoms were found in 247 (89.49) patients, reported at table 1. Preoperative CA 125 serum level had a median value of 45.5 (19.6-90.0), varying from 6.0 to 975.0. Postoperative median value was 15.0 (9.0-25.0), varying from 4.0 to 155.0. Diagnosis was made by laparoscopic surgery in 79.86% (234) of cases, against 20.14% (59) by laparotomy. They were classified as minimal or mild stages (I and II) in 5.80% (17) of procedures, while 94.20% (276) had moderate or severe stages (III and IV). Thirty patients (10.3%) had also deep infiltrating endometriosis. Extra ovarian lesions are shown in table 2, according to their prevalence.
|
Symptom |
N |
% |
|
Dysmenorrhoea |
212 |
76.81 |
|
Dyspareunia |
116 |
42.49 |
|
Acyclic pelvic pain |
108 |
39.13 |
|
Obstipation |
88 |
32.1 |
|
Infertility |
57 |
20.88 |
|
Dyschezia |
14 |
5.07 |
|
Tenesmus |
5 |
1.81 |
|
Hematuria (or urinary symptoms) |
5 |
1.81 |
Table 1: Symptoms reported by patients with ovarian endometriosis.
N= absolute number; %= percentage.
|
Location |
N |
% |
|
Peritoneum |
45 |
16.25 |
|
Bowel |
14 |
5.05 |
|
Fallopian tube |
13 |
4.69 |
|
Rectovaginal septum |
12 |
4.33 |
|
Bladder |
5 |
1.81 |
|
Appendix |
5 |
1.81 |
|
Ureters |
2 |
0.72 |
|
Vagina |
1 |
0.36 |
Table 2: Extra ovarian endometriotic lesions found during surgical procedure.
N= absolute number; %= percentage.
Mean size of endometriomas was 4.8cm (±2.1) and 70.05% (205) of patients had cysts larger than 4cm. There was bilateral involvement in 27.80% (82) of times. Drainage was made in 34.04% (128) of affected ovaries; oophorectomy in 18.67% (70) of them; and cystectomy in 47.73% (179). For clinical treatment, postoperative use of GnRH agonist was found in 40.07% (133) of charts. In 83.39% of times, it was reported some kind of hormonal therapy: 40.43% (93) used combined hormonal contraception and 59.57% (137) isolated progestogen, 4.61% (13) of which used hormone-releasing IUD. Regarding fertility, 7.47% (21) of patients with pregnancy desire were sent to a specialized center in human reproduction for follow up. Regarding follow up, median time was 74.16 months (40.26-112.73) and 58.11% (172) of patients had more than 5 years of follow up. From 255 patients who had clinical treatment discriminated, 50.98% (130) stopped it at some point after surgery. Median time without treatment was 18.0 months (8.5-37.50).
There were 375 affected ovaries that underwent surgical treatment. In 9.87% (37) of them there was persistence of the endometriomas, with median time of persistence of 9.63 months (5.31-13.65). In 20.27% (77) there was recurrence in the long-term. Median time of recurrence presentation was 36.1 months (20.13-72.63). Altogether, 29.73% (114) of endometriomas had either persistence or recurrence. When oophorectomy was performed, twenty patients (28.9%) had recurrence in contralateral ovary. After logistic regression model was used, “drainage of endometrioma” and “postoperative use of GnRH agonist” were the only independent variables related to persistence, adjusted by “cyst size” and “interruption of clinical treatment” (Table 3).
|
Variable |
Univariate analysis |
Multiple analysis |
|
|
OR (CI); “p” value |
OR (CI); “p” value |
|
Drainage |
7.98 (3.92-16.27); 0.000 |
9.09 (3.29-25.12); 0.000 |
|
Postoperative GnRH agonist |
5.49(2.70-11.17); 0.000 |
7.16(2.53-20.25); 0.000 |
|
Cyst size |
1.13(0.99-1.28); 0.072 |
1.06(0.91-1.23); 0.447 |
|
Interruption of clinical treatment |
0.72(0.37-1.40); 0.339 |
0.53(0.23-1.24); 0.147 |
Table 3: Logistic regression model for persistence of ovarian endometriomas.
OR: Odds Ratio; CI: Confidence Interval; LR chi2: 59.11 (Likelihood ratio of R2); Godness-of-fit Hosmer-Lemeshow Test: 76.33
Only the independent variable “interruption of clinical treatment” was related to recurrence of disease, adjusted by “Infertility”, “cyst size in centimeters (cm)”, “bilaterality of endometriosis cysts” and “drainage surgical technique” (Table 4).
|
Variable |
Univariate analysis |
Multiple analysis |
|
|
OR (CI); “p” value |
OR (CI); “p” value |
|
Interruption of clinical treatment |
5.10 (2.76-9.41); 0.000 |
5.93 (2.81 -12.53); 0.000 |
|
Infertility |
2.18(1.30-3.66); 0.003 |
1.57(0.80-3.12); 0.192 |
|
Bilaterality |
0.64 (0.39-1.06); 0.081 |
0.74(0.39-1.40); 0.738 |
|
Cyst size (cm) |
1.09(0.97-1.22); 0.131 |
1.09(0.96 -1.24); 0.188 |
|
Drainage of the cyst |
1.29(0.78-2.12); 0.311 |
1.66 (0.87-3.16); 0.125 |
Table 4: Logistic regression model for recurrence of ovarian endometriomas.
OR: Odds Ratio; CI: Confidence Interval; cm: Centimeters; LR chi2: 35.41 (Likelihood ratio of R2); Godness-of-fit Hosmer-Lemeshow Test: 78.00
The biological gradient was tested using “time elapsed to recurrence” as an independent variable and an inverse correlation was observed between “duration of clinical treatment interruption” and “time elapsed to recurrence” [OR: 0.51(0.03 - 1.00); p= 0.039]. Also, drainage technique was associated to less time to recurrence [OR: -24.15 (-45.50; -2.81); p=0.027] adjusted by “interruption of clinical treatment” (categorical variable).
DISCUSSION
Despite many studies about endometriosis in the literature, few are known about the physiology and risk factors involved in recurrence after surgical treatment. That can be partially explained by multiple variables possibly involved and lack of an exact definition of recurrence, once some authors define as the return of symptoms and others as the return of suspicious images [16]. Ultrasonography may seem more objective and feasible for comparison. Some authors establish as recurrence ovarian endometriomas equal or larger than 20mm detected on transvaginal ultrasonography [13,17]. Yet, there is no definition of persistence, as well as its distinction from recurrence. In this study, these two classifications were separated because, in our understanding, lesions diagnosed soon after surgery are thought to be related to the procedure itself. When analysed together, persistent lesions can interfere with analysis of long-term recurrence and its risk factors.
Some studies show association of recurrence with BMI, age at surgery, cyst size, bilateral involvement and presence of dysmenorrhea [1,18]. Nonetheless, there is no consensus about them as risk factors. Inverse association with BMI, for instance, is found at a meta-analysis of eleven papers, suggesting that obese patients had lower chance of recurrence [19]. However, some authors associate higher BMI with the return of lesions, probably due to technical difficulties while operating obese patients [1].
Populational characteristics may interfere with research results. In Brazil, obesity increased 67.8% between 2006 and 2018, with obese women accounting for 20.7% of population [20]. In this study, more than 53% of patients were overweight or obese. Still, there was no association between BMI and persistence or recurrence. Similarly, larger cysts are thought to raise chance of recurrence, once it can be difficult to completely remove them during surgery [17]. However, we found no association between cyst size and bilateral involvement with recurrence or persistence. Inverse association of age during surgery and endometrioma recurrence has been consistently mentioned in previous studies [1]. Some authors believe that higher serum levels of estrogen in younger patients may induce aggressive forms of endometriosis; others suggest that they have relatively longer reproductive period [1,17,21]. Oppositely, studies with teenagers show that recurrence rate after surgery in this population is lower than compared with adults [21,22]. In our analysis, age was not correlated with recurrence or persistence. Nonetheless, mean age was 38.6 years old, which suggests that our patients were already relatively older.
As for surgical techniques, drainage was performed in more than 30% of procedures. Although it may preserve ovarian reserve, it is not the gold standard for treatment of ovarian endometriomas [16]. Whenever possible, laparoscopic cystectomy should be the first choice, since it can reduce recurrence, raise chance of natural pregnancy and diminish chronic pelvic pain [4,14,23]. Once the cyst wall is tightly adherent to the ovary, complete resection can sometimes be difficult to perform. If that is the case, it can either lead to destruction of healthy ovarian tissue; or partial resection can leave endometriotic cells at place, raising the chance of recurrence [23].
There are no studies about postoperative persistence of ovarian endometriomas as well as its definition in the literature. We found 9.87% of persistent lesions, which we defined as short-term imaging findings after surgical procedures. Although its evaluation was impaired because of low number of cases, they can reflect incomplete surgical treatment, possibly due to technical difficulties. Incomplete removal of the cyst allows endometriotic lesions to arise in the same location, as well as maintenance of pain symptoms [14]. That explains the association of drainage with persistence in our findings. Correlatively, use of GnRH agonist after surgery was also associated with persistence, possibly due to a selection bias. They are frequently chosen as clinical treatment after incomplete surgical resection, as they suppress ovarian activity and induce hipoestrogenic state. That said, its association with persistence may indirectly infer they have been chosen for patients whose surgery did not eradicated every visible endometriotic foci or in those with advanced disease in which the procedure was difficult to be performed properly.
There is no consensus in the literature about GnRH agonists efficacy over different stages of the disease or differences among available regimens and routes of administration. Also, evidence is limited regarding dosage or duration of treatment [12]. Six-months therapy after surgery seems to have a beneficial impact on recurrence rate when compared with a 3-months treatment [11,21]. In this study, time of therapy was not discriminated and therefore we could not compare these regimen outcomes.
Recurrence rate (20.27%) was lower than those reported in the literature, especially considering our mean time of follow up of 74 months and the fact that more than 50% of our patients were followed for more than 5 years. A 2008 systematic review estimated a recurrence rate of 21.5% in 2 years and 40-50% in 5 years after the first surgical procedure [24]. To our knowledge, there are no studies reporting a follow up time greater than five years. Our findings can therefore reflect the treatment at a specialized centre, where experienced professionals perform the surgeries and hormonal therapy after the procedure is mandatory.
When analysing factors associated with recurrence, only “interval without clinical treatment” has shown positive correlation with statistical significance. Endometriosis is a chronic progressive inflammatory disease that recurs after surgery and requires maximum use of medical treatment to prevent the return of lesions and avoid repeated surgeries [24]. Even after effective procedures, microscopic foci may persist. Consequently, patients that stop treatment at some point are susceptible to regrowth of residual lesions, even after unilateral oophorectomy, in accordance with contralateral recurrence found in these cases. That is shown in other studies, reaffirming the necessity of clinical therapy, even after unilateral oophorectomy, especially if contralateral adhesions are present, as they raise chance of recurrence [25].
Current postoperative hormonal treatments include GnRH agonists, progestins and combined oral contraceptives. Their use intent to suppress proliferation of endometriotic implants and reduce adhesion formation [24]. Hence, they reduce recurrence significantly, even if it depends on time of use and patient’s adherence to treatment [11,24]. Likewise, their efficacies are not well compared in the context of endometriomas, as well as there is no consensus in international recommendations [11,15,21]. Combined oral contraceptives have a wide range of formulations, dosages and routes of administration and there are no trials that compare these differences on the effectiveness of treating endometriosis [11]. However, there is evidence that continuous regimen can reduce 88% of recurrence risk when compared with no treatment. Although cyclic regimen also seems to be efficient on preventing recurrence, it is less effective on controlling pain symptoms [11]. Comparatively, irregular use of hormonal therapy reduces considerably their benefit. Also, its effect is not cumulative, loosing efficacy as soon as it is interrupted [11]. In this study, more than 50% of patients interrupted clinical treatment at some point, remaining on average 18 months without any kind of hormonal therapy. Although reasons for discontinuation were not evaluated, up to 5.5% of women quit the use of hormonal contraceptives due to secondary effects [11].
There is no evidence of superiority among hormonal treatments in controlling pain symptoms or in the recurrence of endometriomas [11]. However, aside the formulation chosen, it is important to maintain follow up and assure both clinical therapy and patient’s adherence to the treatment, since its interruption can be the best predictor of recurrence. In this context, hormonal treatment after surgery is imperative because it reduces endometrial cells proliferation and inhibits ovulation, diminishing chronic pain and reducing chance of arising lesions. The opposite effect is found in patients that discontinue treatment, even for a transient period of time. Therefore, it is clear that maintenance of postoperative clinical treatment is essential for preventing recurrence of endometriomas, which is found in other studies [10,13,26,27]. It is indispensable to inform patients about it, as well as the need for their adherence in the success of treatment. Likewise, incomplete surgical procedures should be avoided whenever possible and more thorough techniques should be tested in order to limit residual endometriotic cells, aiming to diminish chance of persistence.
The limitation of this study regards to its observational design, therefore it is not possible to address causality. However, within this context, it matches some Hill’s and GRADE's criteria towards the strength of the findings, such as: the effect size (almost 6 times regarding recurrence and 9 times regarding persistence); consistency [10,13,26,27], temporality (cohort study followed participants prospectively) and dose response relationship (inversely correlating “duration of clinical treatment interruption” and “time elapsed to recurrence”) [28,29]. Thus, reinforcing the hypothesis for possible risk of endometriosis recurrence when the ovarian inhibition is not sustained after surgical treatment, despite its technique and other lesions characteristics. Also, the reference centre from which data was collected does not have a fertility centre and therefore the impact of chirurgical intervention on fertility status of affected women was not evaluated. Nonetheless, to our knowledge, this is the only study to address differences between cysts persistence and recurrence, also correlating the former with incomplete surgical technique and adjuvant treatment of GnRH analogue inferring technical difficulties.
CONCLUSION
Ovarian endometriomas had a persistence rate of 9.87% and recurrence rate of 20.27% after surgical treatment. Drainage of ovarian endometrioma and complementary treatment with GnRH agonists after surgery were the only predictors associated with persistence. Although this outcome is not defined or analyzed in the literature, it can be of great value to evaluate postoperative response. Interruption of postoperative clinical treatment was the only factor associated with lesion recurrence.
REFERENCES
- Han S, Lee H, Kim S, Joo J, Suh D, et al. (2018) Risk factors related to the recurrence of endometrioma in patients with long-term postoperative medical therapy. Ginekol Pol 89: 611-617.
- Zheng Q, Mao H, Xu Y, Zhao J, Wei X, et al. (2016) Can postoperative GnRH agonist treatment prevent endometriosis recurrence? A meta-analysis. Arch Gynecol Obstet 294: 201-207.
- Middleton LJ, Daniels JP, Weckesser A, Bhattacharya S (2017) Preventing recurrence of endometriosis by means of long-acting progestogen therapy (PRE-EMPT): Report of an internal pilot, multi-arm, randomised controlled trial incorporating flexible entry design and adaption of design based on feasibility of recruitment. Trials 18: 121.
- Mereu L, Florio P, Carri G, Pontis A, Petraglia F, et al. (2012) Clinical outcomes associated with surgical treatment of endometrioma coupled with resection of the posterior broad ligament. Int J Gynaecol Obstet 116: 57-60.
- Nisolle M, Donnez J (1997) Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril 68: 585-596.
- Johnson MC, Torres M, Alves A, Bacallao K, Fuentes A, et al. (2005) Augmented cell survival in eutopic endometrium from women with endometriosis: Expression of c-myc, TGF-beta1 and bax genes. Reprod Biol Endocrinol 3: 45.
- Dai Y, Zhou Y, Zhang X, Xue M, Sun P, et al. (2018) Factors associated with deep infiltrating endometriosis versus ovarian endometrioma in China: A subgroup analysis from the FEELING study. BMC Women's Health 18: 1-9.
- Lopez ACS, Santos LLR, Ramos JFD, Yatabe S, Lopes RGC, et al. (2000) Tratamento Videolaparoscópico de Endometriomas Ovarianos. Rev Bras Ginecol Obstet 22: 615-618.
- Vercellini P, Chapron C, De Giorgi O, Consonni D, Frontino G (2003) Coagulation or excision of ovarian endometriomas? Am J Obstet Gynecol 188: 606-610.
- Vercellini P, DE Matteis S, Somigliana E, Buggio L, Frattaruolo MP, et al. (2013) Long-term adjuvant therapy for the prevention of postoperative endometrioma recurrence: A systematic review and meta-analysis. Acta Obstet Gynecol Scand 92: 8-16.
- Geoffron S, Legendre G, Daraï E, Chabbert-Buffet N (2017) Traitement médical de l’endométriose: Prise en charge de la douleur et de l’évolution des lésions par traitement hormonal et perspectives thérape La Presse Médicale. 46: 1199-1211.
- Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D'Hooghe T, et al. (2014) ESHRE guideline: Management of women with endometriosis. Hum Reprod 29: 400-412.
- De Carvalho MSR, Pereira AMG, Martins JA, Lopes RCG (2014) Fatores preditores de recorrência do endometrioma ovariano após tratamento laparoscópi Rev Bras Ginecol Obstet 37: 77-81.
- Selçuk I, Bozda? G (2013) Recurrence of endometriosis; risk factors, mechanisms and biomarkers; review of the literature. J Turk Ger Gynecol Assoc 14: 98-103.
- Chen YJ, Hsu TF, Huang BS, Tsai HW, Chang YH, et al. (2017) Postoperative maintenance levonorgestrel-releasing intrauterine system and endometrioma recurrence: A randomized controlled study. Am J Obstet Gynecol 216: 582.
- Rubod C, dit Gautier EJ, Yazbeck C (2018) Traitement chirurgical des endométri Modalités et résultats en termes de douleur, fertilité et récidive des techniques chirurgicales et de ses alternatives. RPC Endométriose CNGOF-HAS. Gynécologie Obstétrique Fertilité & Sénologie 46: 278-289.
- Küçükba? M, Kurek Eken M, ?lhan G, ?enol T, Herkilo?lu D, et al. (2018) Which factors are associated with the recurrence of endometrioma after cystectomy? J Obstet Gynaecol 38: 372-376.
- Li XY, Chao XP, Leng JH, Zhang W, Zhang JJ, et al. (2019) Risk factors for postoperative recurrence of ovarian endometriosis: Long-term follow-up of 358 women. J Ovarian Res 12: 79.
- Liu Y, Zhang W (2017) Association between body mass index and endometriosis risk: A meta-analysis. Oncotarget 8: 46928-46936.
- Ministry of Health (2019) Brasileiros atingem maior índice de obesidade nos últimos treze anos. Ministry of Health, Brazil.
- Nowak-Psiorz I, Cie?wie? SM, Brodowska A, Starczewski A (2019) Treatment of ovarian endometrial cysts in the context of recurrence and fertility. Adv Clin Exp Med 28: 407-413.
- Lee SY, Kim ML, Seong SJ, Bae JW, Cho YJ (2017) Recurrence of Ovarian Endometrioma in Adolescents after Conservative, Laparoscopic Cyst Enucleation. J Pediatr Adolesc Gynecol 30: 228-233.
- Shaltout MF, Elsheikhah A, Maged AM, Elsherbini MM, Zaki SS, et al. (2019) A randomized controlled trial of a new technique for laparoscopic management of ovarian endometriosis preventing recurrence and keeping ovarian reserve. J Ovarian Res 12: 66.
- Guo SW (2009) Recurrence of endometriosis and its control. Hum Reprod Update 15: 441-461.
- Hidari T, Hirata T, Arakawa T, Koga K, Neriishi K, et al. (2019) Contralateral ovarian endometrioma recurrence after unilateral salpingo-oophorectomy. BMC Womens Health 19: 59.
- Vercellini P, Somigliana E, Viganò P, De Matteis S, Barbara G, et al. (2010) Post-operative endometriosis recurrence: A plea for prevention based on pathogenetic, epidemiological and clinical evidence. Reprod Biomed Online 21: 259-265.
- Somigliana E, Vercellini P, Vigano P, Benaglia L, Busnelli A, et al. (2014) Postoperative medical therapy after surgical treatment of endometriosis: From adjuvant therapy to tertiary prevention. J Minim Invasive Gynecol 21: 328-334.
- Hill AB (1965) The Environment and Disease: Association or Causation? Proc R Soc Med 58: 295-300.
- Schünemann H, Hill S, Guyatt G, Akl EA, Ahmed F (2011) The GRADE approach and Bradford Hill's criteria for causation. J Epidemiol Community Health 65: 392-395.
Citation: Salgado IMA, Pereira AMG, Lopes RGC, Davi SD, Pinheiro DJPC, et al. (2020) Persistence and Recurrence of Ovarian Endometrioma. J Reprod Med Gynecol Obstet 5: 038.
Copyright: © 2020 Salgado IMA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

