
PrEP Education and Awareness Building through an Intervention for African-Americans Reporting both Condomless Sex and Substance Use During an Emergency Department Visit
*Corresponding Author(s):
Mandy J. HillDepartment Of Emergency Medicine, University Of Texas Health, McGovern Medical School, Houston, United States
Tel:+713-500-7661,
Email:Mandy.J.Hill@uth.tmc.edu
Abstract
Background: Pre-exposure prophylaxis (PrEP) provides women with an effective tool to prevent HIV. Uptake among African American women remains low while new HIV diagnoses continue to rise.
Methods: In a single-armed study, we enrolled African American women (n=14) ages 20-29 years. They acknowledged recent substance use and sex during an emergency department visit. We delivered the ‘Increasing PrEP uptake’ (iPrEP), an intervention that uses brief messages to raise HIV risk awareness related to substance use and sex, on tablet devices.
Results: Most women completed high school/GED (n=12/14), reported a monthly income below the federal poverty line (n=9/14), were employed (n=9/14), and had a primary partner (n=13/14). Three women reported sex with a recent casual partner. Most women reported substance use within two hours of condomless sex (n=9/14) and willingness to use PrEP (n=9/14).
Conclusion: Our study suggests iPrEP is potentially associated with PrEP willingness among sexually-active African American women who use substances.
Keywords
African American women; HIV; Pre-exposure prophylaxis (PrEP) education; Risk awareness; Substance use
INTRODUCTION
There are higher rates of new HIV diagnoses among African American women than among women from other racial and ethnic groups [1,2]. Condom less sex and substance use may compound that population risk [3]. Houston, TX ranks 11th in the nation for HIV incidence rates, of which 36% were women, and 71% of those were African American [4]. Risky sexual behavior in Texas is common: 88% of African American women contract HIV through condomless heterosexual sex [5]. Substance use rates are 13.4% for injection drug use and 32.1% for non-injection drug use [6]. Substance use promotes heightened sexual risk taking, substantiating the public health need of prioritizing HIV prevention interventions for this population.
Truvada, an antiretroviral medication, is FDA approved for use as pre-exposure prophylaxis (PrEP) to prevent HIV transmission to vulnerable HIV negative men and women. PrEP can be a key component of a comprehensive HIV prevention strategy that can be controlled by women. When levels of PrEP adherence are high, oral PrEP demonstrates high efficacy among women; however, women with low PrEP adherence experience low efficacy [7,8]. Yet, PrEP use among African American women remains low [9-12].Through an evaluation of a national pharmacy database that accounts for 39% of all Truvada prescriptions, researchers explored PrEP utilization between 2012 and 2015. Researchers identified nearly 50,000 people who started PrEP and only 21% were women [13]. Further, 74% of PrEP prescriptions were among Whites, 12% Latinos, and 10% African Americans. The majority of PrEP prescriptions were written for non-Hispanic White patients; far fewer Latinos and African Americans received prescriptions. In addition to continued risk of contracting HIV, low PrEP uptake among women curtails the ability of HIV prevention researchers to quantify efficacy estimates of this approach for addressing HIV as a public health problem for women.
Barriers to PrEP uptake has been explored. An abstract of a cross-sectional study using a convenience sample of ED patients at New York City hospitals demonstrated that Latino patients (17.6%) reported PrEP knowledge more than African Americans (8.8%) and patients who designated their race as ‘other’ (12.4%) [14]. Cost has been established as a major barrier to PrEP uptake. However, recent changes in guidelines for insurance companies to cover PrEP will potentially overcome this barrier for insured populations. Known behavioral, social, and structural barriers to PrEP uptake that are specific to women have been reported [15]. Evidence from a few studies suggest low perceived risk and concerns about side effects are barriers to PrEP uptake. In a qualitative study of PrEP interest in a sample of 114 women, Carley et al. reported an alignment between low self-reported condom use and low perceptions of HIV risk. Specifically, 84.7% of participants who self-reported low condom use perceived themselves at low risk for HIV. In the same study, merely 7.4% of enrolled women maintained interest in PrEP and believed they could adhere to a daily PrEP regimen after learning of the side effects. None of the women enrolled who perceived themselves to be at medium or high risk for HIV upheld an interest in PrEP [16].
African Americans are two times more likely to consider the emergency departments (ED) as their medical home than Non-Hispanic Whites [17]. Thus, EDs could serve as an access point to increase African American women’s awareness and potentially access to PrEP for HIV prevention. Interventions that can both feasibly integrate into the ED patient flow and increase PrEP uptake among substance-using African American women can help protect this vulnerable populations from contracting HIV.
The aim of this article is to investigate whether African American women, who acknowledged both condomless sex and substance use, were willing to take PrEP after receiving the intervention in ED.
METHODS
Research design
We developed a brief, intervention delivered on a tablet device as a prospective, single-arm quantitative pilot study.
Study population
Fourteen African American women between 20 and 29 years of age who reported substance use and condom less sexual activity within the last three months were recruited from the EDs of two teaching hospitals. Any substance use (including the use of alcohol, marijuana, cocaine, amphetamine, methamphetamine, benzodiazepines, barbiturates, and/or PCP)) and at least one sexual encounter in the last three months qualified the participant for eligibility in the study.
Study setting
Recruitment sites included two hospital-based EDs, one privately-funded the other publicly-funded and the other publicly-funded Memorial-Hermann – Texas Medical Center, the private hospital’s ED, has a patient volume of approximately 72,000 visits each year [18]. Lyndon B. Johnson hospital of the Harris Health System, the public, acute care hospital, has a patient volume exceeding 70,000 visits each year [19,20].
Procedure
The study was approved by the institutional review board at UT-Health (HSC-MS-15-0910). Patients seeking treatment in the ED were screened for eligibility through approved access to the patient’s medical record and collection of de-identified data for screening purposes. The inclusion criteria that determined eligibility for the study were: 1) African American race, 2) female sex and gender (cisgender), 3) a social history with reported sexual activity and substance use within the last three months, and 4) low acuity (1-3) based on the 5-point emergency severity index [21]. Electronic medical records were screened by trained researchers to identify eligible participants.
Eligible participants were recruited by trained members of the research team from the ED waiting room or from private patient rooms. Patients who were recruited from the ED waiting room were taken to a private designated area prior to initiation of the enrollment process.
Researchers initiated the enrollment period (7 months) with eligible participants in a private area, explaining why they were approached (i.e. meeting the eligibility criteria) for participation and providing a summary of the study’s purpose and procedures. The informed consent was discussed and the risks and benefits of study participation were explained. Potential participants were given time to read through the consent form and decide whether or not they wanted to participate. For those who chose not to participate, the researcher inquired about the reason and those reasons were recorded. Those who chose to participate signed the consent form. One copy of the consent form was given to the participant and a second copy was added to the patient’s medical record.
Participants were given the tablet device to self-administer the intervention. The intervention instrument included 99-items. At the conclusion of the survey, the participant showed the final screen to the researcher, ensuring that participation was complete. At that time, the researcher provided the participant with a $25 gift card and study participation concluded.
Intervention
The HIV Prevention Trials Network (HPTN 073) study developed a baseline instrument for African American men who have sex with men (MSM) to assess structural and mental health factors predicting PrEP uptake and adherence [22-24]. They used a counseling strategy to promote PrEP use and supported clients through referrals to related services, resulting in a 79% PrEP acceptance rate among enrollees [25]. Based on an adaptation of the HPTN 073 study, a novel intervention, ‘Increasing PrEP uptake’ (iPrEP), was developed. The adaptation plan for the survey tool focused on the content and format of the HPTN 073 baseline instrument. After adapting the survey tool, it was packaged as an innovative intervention approach with a novel delivery platform using a tablet device. The two aims were: 1) to adapt an intervention that effectively promoted PrEP uptake among MSM for use among African American women and 2) to measure and encourage willingness of African American women seeking healthcare in an ED to use PrEP for HIV prevention.
Socio-demographic variables
The study assessed demographics including, age, education, sexual orientation, household income (monthly), and employment status.
Relationship characteristics
Among women with primary partners, we evaluated relationship type and relationship length. Sexual experiences with casual partners were assessed under sexual behaviors.
Sexual behaviors
Sexual behaviors of participants were assessed in four areas: knowledge of partner’s HIV status, engagement in transactional sex (engagement in the exchange of sex for resources including money and illicit substances), recent sex with a casual partner, and recent oral sex or tribbing/scissoring (rubbing your vaginas against each other to stimulate the clitoris) with a woman.
Substance-using behaviors
Substance use behaviors were evaluated in three areas: frequency of substance use, concurrent substance use and condomless sex, and concurrent substance use and sex with condoms.
The primary outcome of the study, willingness to take PrEP, was measured with one question, which read, ‘Would you be willing to take PrEP to reduce your own risk of getting HIV?’ The 3-item response format allowed participants to answer ‘yes’, ‘no’, or ‘unsure’.
Screening
A total of 261 potentially eligible women seeking care in either a public or private ED were screened and assessed for eligibility (Figure 1). Reasons for screen failures included: 1) failure to report current substance use, 2) presenting complaint acuity level of four or higher (indicating a serious health condition), 3) classified as ineligible based on race, age or gender, 4) failure to locate eligible participant, and 5) patient discharged between screening and recruitment effort. The reasons for refusal to participate for most eligible women were time constraint.
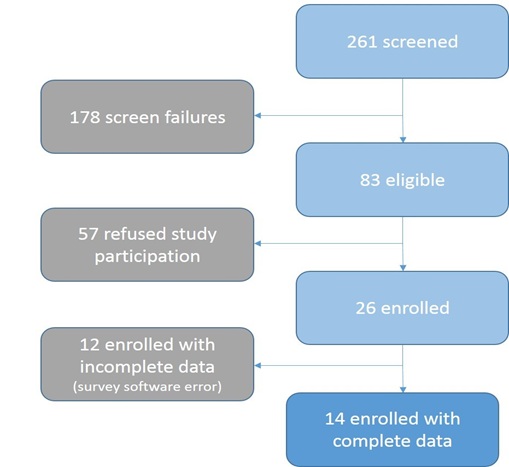 Figure 1: Flow diagram of enrollment cascade.
Figure 1: Flow diagram of enrollment cascade.
Statistical analytic methods
Qualtrics XM software was used to administer PrEP. A frequency analysis using IBM SPSS 21.0 was performed on demographics, relationship characteristics, substance use and sexual behaviors.
RESULTS
Twenty-six women were enrolled and included in the study. Fourteen enrolled women completed the survey. The following results are reported for the 14 women. All but two participants (12/14) completed high school, received a GED, or had some college education. Nine women (9/14) were heterosexual and four women (4/14) reported having had sex with both men and women. Nine women (9/14) were employed. One of the employed women (1/14) but reported a monthly household income that was below the federal poverty line for one person (1/14), as she had a household income of $1000 per month or less (Table 1).
|
Socio-demographic variables |
Subcategories |
|
N |
% |
|
Age |
20 |
|
3 |
21.4 |
|
|
23 |
|
3 |
21.4 |
|
24 |
|
2 |
14.3 |
|
|
26 |
|
1 |
7.1 |
|
|
27 |
|
4 |
28.6 |
|
|
29 |
|
1 |
7.1 |
|
|
Education |
Some high school education |
|
2 |
14.3 |
|
|
High school education or GED |
|
6 |
42.9 |
|
Some College |
|
5 |
35.7 |
|
|
Graduate education |
|
1 |
7.1 |
|
|
Sexual orientation* |
|
|
||
|
|
Heterosexual |
|
9 |
64.3 |
|
|
Bisexual |
|
2 |
14.3 |
|
|
Woman who has sex with women and men |
|
2 |
14.3 |
|
|
Missing |
|
1 |
7.1 |
|
Household Income (monthly) |
|
|
||
|
|
Less than $500 |
|
5 |
35.7 |
|
|
Between $501 and $1,000 |
|
4 |
28.6 |
|
|
Between $1,001 and $1,500 |
|
2 |
14.3 |
|
|
Between $1,501 and $2,000 |
|
2 |
14.3 |
|
|
$2,001 or more |
|
1 |
7.1 |
|
|
|
Employment status |
||
|
|
Employed |
|
9 |
64.3 |
|
|
Unemployed |
|
5 |
35.7 |
|
If unemployed, reason for unemployment |
|
|
||
|
|
Stay at home Mom |
|
2 |
40 |
|
|
Cannot find a job |
|
2 |
40 |
|
|
Don’t know |
|
1 |
20 |
Legend: * - refers to a missing variable. 1 subject did not report sexual orientation.
Percentages may not add up to 100 due to rounding.
Table 1: Socio-Demographics of the study sample (N=14).
Relationship characteristics, sex and substance use behaviors are shown in Table 2. All but one of the women (13/14) had a primary partner and ten participants (10/14) reported that their relationship had lasted more than eight months. Only one woman (1/14) reported that her partner was HIV positive, while twelve others (12/14) believed their partner to be negative. Only one woman (1/14) reported engaging in transactional sex, one (1/14) reported oral sex or tribbing/scissoring with a woman in last 3 months. Three (3/14) reported recent sex with casual partners. Substance use within the last three months ranged from one time to daily. However, over half of women (9/14) enrolled reported substance use within two hours before or after engaging in condomless sex. A similar number of women (8/14) reported substance use within two hours of engaging in sex with condoms.
|
Variables |
Response Options |
N |
% |
|
Relationship type |
|||
|
Primary partner |
Yes |
13 |
92.9 |
|
No |
1 |
7.1 |
|
|
Length of relationship |
|||
|
Less than 1 month |
1 |
7.1 |
|
|
1-4 months |
2 |
14.3 |
|
|
5-7 months |
1 |
7.1 |
|
|
8 months or more |
10 |
71 |
|
|
Sexual behaviors |
|||
|
HIV status of primary partner |
|||
|
HIV negative |
12 |
85.7 |
|
|
HIV positive |
1 |
7.1 |
|
|
Unsure or don’t know |
1 |
7.1 |
|
|
Transactional sex |
|||
|
(Gave/received goods for sex) |
|||
|
Yes |
1 |
7.1 |
|
|
No |
13 |
92.9 |
|
|
Sex with casual partner in last 3 months |
|||
|
Yes |
3 |
21.4 |
|
|
No |
11 |
78.6 |
|
|
Oral sex or tribbing/scissoring with a woman in last 3 months |
|||
|
Yes |
1 |
7.1 |
|
|
No |
13 |
92.9 |
|
|
Substance use behaviors |
|||
|
Frequency of substance use in the last 3 months |
|||
|
Once |
1 |
7.1 |
|
|
A few days a month (1-2 days/month) |
3 |
21.4 |
|
|
Several days a month (3-4 days/month) |
3 |
21.4 |
|
|
A few days a week (1-2 days a week) |
1 |
7.1 |
|
|
Several days a week (3-4 days a week) |
3 |
21.4 |
|
|
Daily |
3 |
21.4 |
|
|
Substance use within 2 hours before or during condomless sex |
|||
|
Yes |
9 |
64.3 |
|
|
No |
5 |
35.7 |
|
|
Substance use within 2 hours before or during sex with condoms |
|||
|
Yes |
8 |
57.1 |
|
|
No |
1 |
7.1 |
|
|
Missing |
5 |
35.7 |
|
aLegend: Percentages may not add up to 100 due to rounding
Table 2: Summary of participant responses on relationships, sex and substance use.
After receiving the educational messages that were embedded in the survey, participants were asked whether they would be willing to take PrEP. Nine women (9/14) said yes, one (1/14) said no, and four (4/14) were unsure.
DISCUSSION
Brief education-based interventions can feasibly be delivered on a tablet device during an ED visit. This intervention strategy can serve as an information source about PrEP while inspiring PrEP uptake among African American women, a population at significant risk for HIV. African Americans are more likely to use the ED to meet their primary care needs than other racial/ethnic groups. ED clinicians commonly treat individuals with sexually transmitted infections [26-29]. The ED is an appropriate clinical environment to engage an at-risk population and broaden the prevention scope to include brief primary prevention strategies.
In order to achieve maximum benefit from an effective HIV prevention strategy for African American women, PrEP should be included. Previous studies have aimed to better understand PrEP awareness among PrEP-eligible women. Findings demonstrated low levels of PrEP awareness and high levels of interest in PrEP [9,11,30]. Those studies used focus groups and traditional qualitative methods that were absent of intervention elements. For the first time, to our knowledge, enrolled women engaged in an intervention designed to enhance their willingness to adopt PrEP for HIV prevention. Willingness to use PrEP could translate to higher uptake rates among people who need it most.
Sexual networks of African American women in the South are often racially homogeneous. African American sexual networks also have a higher prevalence of HIV and STIs when compared to other races and ethnicities [31,32]. Most women enrolled reported concurrent HIV risk behaviors. Some enrolled women engaged in substance use with sex (both with condoms and without).
Despite high vulnerability to HIV to African American women in the South, primarily due to condomless heterosexual sex, PrEP uptake among women remains low [33]. Disparate rates demonstrate potential benefit of PrEP to 500,000 African Americans versus filled prescriptions of only 7,000 between 2015 and 2016 [34]. Increased PrEP uptake among African American women could provide a complementary HIV preventive strategy when condoms fail or are not used [35,36]. Sexually-active African American women who engage in substance use require HIV prevention strategies to overcome vulnerabilities driven by race, gender, sexual network, and sexual behaviors [37]. A prospective assessment of 300 people (57% were African American) attending an HIV clinic in Houston, Texas revealed that only 27% of attendees always used condoms, although 67% perceived personal HIV risk. These findings substantiate challenges in navigating condom use to African American women locally.
LIMITATIONS
A primary limitation of the study is the small sample size. Of the 26 women enrolled, twelve surveys were not usable due to a software error. Due to the sample size, findings cannot be generalized to other groups of African American women who report substance use and sexual activity. In addition, the presence of a single-arm study design limits hypothesis testing. This pilot study should be expanded as a two-armed study using a randomized clinical trial (RCT) method comparing iPrEP to usual care with a larger sample size and at other emergency departments. This was a sample of African American women in which the majority of them reported having sex while under the influence of substances; thus, findings should not be generalized to all African American women. The response format for sexual orientation did not allow participants to select more than one option. This may have obscured distinctions between sexual orientation and sexual practice. Lastly, the population is not representative of the general population in the Houston area; however, they may offer insights about those who seek care in EDs. Participants had varying levels of education and access to health care.
CONCLUSION
The ‘Increasing PrEP uptake’ (iPrEP) intervention was demonstrated to be feasible, associated with willingness to use PrEP using a single-armed approach, and is ready to be tested in a RCT. This pilot study led to a funded randomized controlled trial (RCT) that is actively enrolling participants to assess whether iPrEP is more effective at increasing willingness to take PrEP than usual care among a broader demographic of African American women ages 18-55 years during an ED visit for a non-emergent condition. The RCT will also evaluate whether iPrEP intervention plus a PrEP referral increases visit attendance at the PrEP clinic and promotes PrEP uptake within six months. Findings will further determine feasibility, acceptability and efficacy of the iPrEP as well as determine effect size estimates for a future, large-scale efficacy trial. Public health benefit to communities at risk for HIV may be realized via innovative intervention and referral mechanisms that increase currently low rates of PrEP uptake among at-risk minority populations.
ACKNOWLEDGEMENTS
We acknowledge Erica Hamilton, program manager of the HIV Prevention Trials Network (HPTN) Scholar’s Program, Dr. Darrell Wheeler (PI of HPTN 073), and Dr. Leo Wilton for sharing the HPTN 073 PrEP adherence tool for adaptation in the presented research with Dr. Mandy Hill during her tenure as an HPTN scholar.
FUNDING
This study was funded by the Baylor-UT Health Center for AIDS Research through the National Institutes of Health (AI36211 NIAID PI: Dr. Mandy Hill) and in part by the National Institutes of Health 5K23MH109358-02 (PI: Charlene Flash) for concept and theory development.
CONFLICT OF INTEREST
Dr. Mandy Hill received an investigator sponsored research award from Gilead Sciences, Inc. after the research presented in this paper was conducted in 2019. This industry sponsors manufactures Truvada, the drug used as pre-exposure prophylaxis. Dr. Mandy Hill declares no additional conflicts. Dr. Charlene Flash has no conflicts of interest. Dr. Angela Heads has no conflicts of interest. Dr. Marylou Cardenas-Turanzas has no conflicts of interest. Dr. Richard Grimes has no conflicts of interest.
RESEARCH INVOLVING HUMAN PARTICIPANTS AND/OR ANIMALS
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in the study.
REFERENCES
- HIV and African American women. Gateway to Health Communication and Social Marketing Practice 2015. Centre for Disease Control and Prevention (CDC).
- Adimora AA, Schoenbach VJ, Doherty IA (2006) HIV and African Americans in the southern United States: sexual networks and social context. Sex Transm Dis 33: 39-45.
- Dunkle KL, Wingood GM, Camp CM, DiClemente RJ (2010) Economically motivated relationships and transactional sex among unmarried African American and white women: results from a U.S. national telephone survey. Public Health Rep 125: 90-100.
- AIDS: Summary of Adult Reportable Houston/Harris County Cases (2015) City of Houston Health Department.
- Black Women and HIV in Harris County (2014) Texas Department of State Health and Human Services Program. Department of State Health Services HIV/STD Program: Austin, TX.
- Maxwell JC (2015) Substance Abuse Trends in Texas: June 2015 J. Proceedings of the Community Epidemiology Work Group: 42.
- Escudero DJ, Kerr T, Wood E, Nguyen P, Lurie MN, et al. (2015) Acceptability of HIV Pre-exposure Prophylaxis (PREP) Among People Who Inject Drugs (PWID) in a Canadian Setting. AIDS Behav 19: 752-757.
- Koss CA, Liu AY, Castillo-Mancilla J, Bacchetti P, McHugh C, et al. (2018) Similar tenofovir hair concentrations in men and women after directly observed dosing of tenofovir disoproxil fumarate/emtricitabine: implications for preexposure prophylaxis adherence monitoring. AIDS 32: 2189-2194.
- Flash CA, Stone VE, Mitty JA, Mimiaga MJ, Hall KT, et al. (2014) Perspectives on HIV prevention among urban black women: a potential role for HIV pre-exposure prophylaxis. AIDS Patient Care STDS 28: 635-42.
- Huang YA, Zhu W, Smith DK, Harris N, Hoover KW, et al. (2018) HIV Preexposure Prophylaxis, by Race and Ethnicity - United States, 2014-2016. MMWR Morb Mortal Wkly Rep 67: 1147-1150.
- Auerbach JD, Kinsky S, Brown G, Charles V (2015) Knowledge, attitudes, and likelihood of pre-exposure prophylaxis (PrEP) use among US women at risk of acquiring HIV. AIDS Patient Care STDS 29: 102-110.
- Bush S, Magnuson D, Rawlings MK, Hawkins T, Giler M, et al. (2016) Racial characteristics of FTC/TDF for pre-exposure prophylaxis users in the U.S. Paper. ASM Microbe: Boston, MA.
- Bush S, Ng L, Magnuson D, Piontkowsky D, Giler M, (2016) Significant Uptake of Truvada for Pre-exposure Prophylaxis (PrEP) Utilization in the US in Late 2014 – 1Q2015, IAPAC Treatment, Prevention, and Adherence Conference (June 28-30), Gilead: 01-18.
- Calderon Y, Leider J, Cowan E, Brusalis C, Mantell J, et al. (2013) HIV pre-exposure prophylaxis (PrEP): Knowledge and attitudes among a New York city emergency department patient population. J AIDS Clin Res 4: 94.
- Flash CA, Dale SK, Krakower DS (2017) Pre-exposure prophylaxis for HIV prevention in women: current perspectives. Int J Womens Health 9: 391-401.
- Carley T, Siewert E, Naresh A (2019) Interest in Pre-exposure Prophylaxis (PrEP) for HIV is Limited Among Women in a General Obstetrics & Gynecology Setting. AIDS Behav 23: 2741-2748.
- Brown LE, Burton R, Hyson B, Kakade M, Bhagalia P, et al. (2012) Factors influencing emergency department preference for access to healthcare. West J Emerg Med 13: 410-415.
- University of Texas Health Science Centre at Houston (2016) McGovern Medical School. Hospital Profiles.
- Harris Health System. Lyndon B. Johnson Hospital (2018).
- Harris Health System. Facts and Figures. (2019).
- Agency for Healthcare Research and Quality (AHRQ), Emergency Severity Index (ESI): A Triage Tool for Emergency Departments, U.S.D.o.H.a.H. Services (2012): Washington, DC.
- HIV Prevention Trials Network. HPTN 073: Consistency Between Self-Report and Drug Levels for PrEP Among Black MSM in U.S (2016).
- HIV Prevention Trials Network. HPTN 073: Pre-Exposure Prophylaxis (PrEP) Initiation and Adherence among Black Men who have Sex with Men (BMSM) in Three U.S. Cities. 2016.
- Wheeler DP, Fields S, Nelson L, Wilton L, Hightow-Weidman L, et al. (2016) PrEP Uptake and Use by Black Men Who Have Sex With Men in 3 US Cities, in Predictors of PrEP Coverage and Uptake, C.o.R.a.O.I. (CROI). State University of New York at Albany: Boston, MA. J Int AIDS Soc 22.
- Wilton L, Nelson L, Beauchamp G, Magnus M, Shoptaw M, et al. (2017) Structural barriers, mental health, and PrEP initiation/non-initiation and adherence among Black MSM in 3 U.S. cities - HPTN 073 study. J Int AIDS Soc 22.
- Bernstein E, Goldfrank LR, Kellerman AL, Hargarten SW, Jui J, et al. (1994) A public health approach to emergency medicine: preparing for the twenty-first century. Acad Emerg Med 1: 277-86.
- Mehta SD, Rothman RE, Kelen GD, Quinn TC, Zenilman JM (2001) Unsuspected gonorrhea and chlamydia in patients of an urban adult emergency department: a critical population for STD control intervention. Sex Transm Dis 28: 33-9.
- Haukoos JS, Mehta SD, Harvey L, Calderon Y, Rothman RE, et al. (2009) Research priorities for human immunodeficiency virus and sexually transmitted infections surveillance, screening, and intervention in emergency departments: consensus-based recommendations. Acad Emerg Med 16: 1096-1102.
- Todd CS, Haase C, Stoner BP (2001) Emergency department screening for asymptomatic sexually transmitted infections. Am J Public Health 91: 461-464.
- Goparaju L, Praschan NC, Jeanpiere LW, Experton LS, Young MA, et al. (2017) Stigma, Partners, Providers and Costs: Potential Barriers to PrEP Uptake among US Women. J AIDS Clin Res 8.
- Adimora AA, Schoenbach VJ (2002) Contextual factors and the black-white disparity in heterosexual HIV transmission. Epidemiology 13: 707-712.
- Adimora AA, Schoenbach VJ, Bonas DM, Martinson FE, Donaldson KH, et al. (2002) Concurrent sexual partnerships among women in the United States. Epidemiology 13: 320-327.
- Patel AS, Goparaju L, Sales JM, Mehta CC, Blackstock OJ, et al. (2019) Brief Report: PrEP Eligibility Among At-Risk Women in the Southern United States: Associated Factors, Awareness, and Acceptability. J Acquir Immune Defic Syndr 80: 527-532.
- Center for Disease Control and Prevention (CDC) (2018) HIV prevention pill not reaching most Americans who could benefit – especially people of color.
- Bowleg L, Valera P, Teti M, Tschann JM (2010) Silences, gestures, and words: nonverbal and verbal communication about HIV/AIDS and condom use in black heterosexual relationships. Health Commun 25: 80-90.
- Flash CA, Adegboyega OO, Yu X, Avalos C, Johnson S, et al (2018) Correlates of Linkage to HIV Preexposure Prophylaxis Among HIV-Testing Clients. J Acquir Immune Defic Syndr 77: 365-372.
- Broaddus M, Owczarzak J, Pacella M, Pinkerton S, Wright C, et al. (2016) Partnership-Level Analysis of African American Women's Risky Sexual Behavior in Main and Non-Main Partnerships. AIDS Behav 20: 2893-2903.
Citation: Hill MJ, Flash CA, Heads A, Turanzas MC, Grimes R (2020) PrEP Education and Awareness Building through an Intervention for African-Americans Reporting both Condomless Sex and Substance Use During an Emergency Department Visit. J AIDS Clin Res Sex Transm Dis 7: 028.
Copyright: © 2020 Mandy J. Hill, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

