
Prophylatic Potentials of Dietary Syzgium malaccence leaves Extracts on the Metabolite Activities and Organosomatic Indices of Clarias gariepinus
*Corresponding Author(s):
Ukwe IOKDepartment Of Fisheries Sand Aquatic Environment, Rivers State University, Nkpolu-Oroworukwuo, Port Harcourt, Rivers State, Nigeria
Email:oyekuotorisaac@gmail.com
Abstract
The potency of Syzgium malaccence as an immune stimulant and a prophylactic was accessed. 40% crude protein fish diet was formulated, and five different feeds were produced using varying concentrations of Syzgium malaccence leave aqueous extracts. (Five hundred grams per liter (500g/l) of water) as follows: 0ml/kg (Do), 25ml/kg (D1), 50ml/kg (D2), 75ml/kg (D3) and 100ml/kg (D4). One hundred and eighty (180) Clarias gariepinus was divided into six groups of ten each in triplicate and were fed the five diets and a known commercise diet (Blue crown) so as to compare the results with that of the known commercial diet. After eight weeks feeding, the experimental fish were exposed to staphylococcus aureus via intraperetoneal injection and observed for one week. Blood samples were collected at the end of weeks 4 and 8 of feeding, and at the end of the post infection period for metabolite [Urea (UR), creatinine (CR), Total protein (TP), Albumin (ALB), Globulim (GLB) and Albumin/Globulin ratio (A/G)] analysis. Organs were also harvested at the end of the post infection period for the determination of the following organosomatic indices: Hepatosomatic index (HSI), Viscerasomatic index (VSI) cistosomatic index (CSI), gonadosomatic index (GSI), Gilosomatic index (GI-SI); spleenosomatic index (SSI) and interperetoneal fat (IF). The result reveals that the diets D1-D4 maintained the functionality of the fish kidney better than the diets Do and BC that negatively altered the functions of the kidney and gills by releasing more UR and CR to the fish blood, at the end of the eight (8) weeks feeding. D1 – D4 unlike Do and BC also prevented the virulence of the S. aureus on the reproductive organs, gills and liver of the fish as seen in the improved GSI, SI, UR and CR.
Keywords
Metabolites; Organosomatic indices Clarias gariepinus; Prophylatic; Syzgium malaccence
Introduction
Fish is a less expensive and significant source of protein, vitamins and other essential nutrients for the body [1], most part of the world especially Africa depend on fish as their source of animal protein [2]. There is serious decline in fish presence in our waters as a result of over fishing arising from high demand for fish and fisheries product, and other anthropogenic activities. Over 65% of our fish stock are either fully exploited or over exploited [3]. Clarias gariepinus is the cheapest source of protein for the middle class citizens in the developing countries especially Nigeria [4].
Aquaculture is the fastest growing sector in agriculture [5], and it stands out as the only alternative to meet-up the high demand of fish and fisheries products. Chemicals are often used by aquaculturist to enhance growth and improve immunostimulant in fish [6], but the use of chemicals is seriously criticized due to bacteria resistance, environmental pollution and pollution of fish flesh [7]. Ukwe and Gabriel [8] stated that the use of herbs and herbal supplement is the best alternative to the use of chemicals because it is cheap, readily available, non-pollutant and achieve same positive results and even more. Most of the used herbs in aquaculture practice include: chromoleana odorata [9], citrus medica [10] and Persea americana [11]. Herbs and herbal products contains primary and secondary phytochemicals [12] and each of these phytochemicals plays one or several roles in sustainable and productive aquaculture [13].
Though fish is a major source of animal protein to man, it’s consumption sometimes lead to contamination and infection as a result of bacterial infections and zoonotic parasites arising from feeds, area of culture and sales point [14,15]. Infected fish most times shows no symptoms, but manifest as zoonotic diseases [16], but they can be identified via haematological and biochemical analysis of the fish blood [17-19] and examination of the fish organs [20].
Metabolitie alterations in the blood of fish is an indication of disease presence [21] or an improved innate immune response in fish [22]. Syzgium malaccence has high phenolic and flavonoid contents in its parts that are antimicrobial with strong antioxidant activities [23,24].
The purpose of this research is to access the potency of aqueous extract of Syzgium malaccence leaves in improving the innate immune response in Clarias gariepinus and it’s prophylactic efficiency in preventing the alteration of the organosomatic indices and metabolites activities in Clarias gariepinus exposed to Staphylococcus aureus.
Materials And Methods
- Study location
The project work was carried out in the laboratory of the Department of Fisheries and Aquatic Environment, Faculty of Agriculture, Rivers State University, Nkpolu-Oroworukwo, Port Harcourt, Rivers State, Nigeria.
- Experimental fish and acclimitization
One hundred and eighty (180) healthy C. gariepinus of mean weight 130-150kg was purchased from Idi-onyana Farms along Abua-Ahoada road in Abua/Odual Local Government Area, Rivers State. The fish was taken to the project site, acclimatization and observation were carried out on the fish for a period of two (2) weeks to access disease present or bruises. During this period the fish was fed to satisfaction with blue crown commercial feed twice daily.
- Source of pathogen
The pathogen staphylococcus aureus was procured from the Department of Microbiology of the Rivers State University, Nkpolu Oroworukwo, Port Harcourt.
- Preparation of syzgium malaccense leave extract
Syzgium malaccense leaves were harvested within Port Harcourt, Rivers State, Nigeria. The leaves were prepared using the method of Ukwe and Jamabo [25]. S. malaccense leaves were harvested, washed clean, pounded to paste, soak in tap water at the concentration of five hundred grams/litre (500g/L) for twelve hours. It was filtered and the filtrate was used immediately.
- Experimental diet
40% crude protein diet was formulated using the following ingredient: Wheat-brand, corn, soyabeans, blood meat, fish meal, lysine, methionine, P. oil, starch and vitamins. Five different diets were produced from the formulated diet using varying concentration of S. malaccense leave aqueous extract at 0ml/g, 25ml/g, 50m/g, 75ml/g and 100ml/g, and labeled Do, D1, D2, D3 and D4 respectively.
- Blue crown feed
The blue crown feed was bought from SAMMIANI CONSULT NIG. LTD, KMI Eneka Junction Igbo Etche Road, Rumukrushi, Obio/Akpor Local Government Area, Rivers State, Nigeria.
- Experimental design
A complete randomized design was used with a total of eighteen (18) experimental tanks.
- Experimental procedure
One hundred and eighty (180) C. gariepinus were distributed into six group of ten in triplicates, and were fed accordingly with Do, D1, D2, D3 and D4 diets, and Blue crown feed (BC). Blood samples were collected at the end of week four (4) and week eight (8) of feeding. After eight weeks of feeding, the experimental fishes were exposed through intraperetonial injection to overnight grown staphylococcus aureus using an injection syringe and 21- guage hypodemic needle at day one (1) and two (2) (twenty four hours interval), and were observed for seven days. Blood samples were taken from the various groups at the end of 4 and 8 weeks feeding and after seven (7) days post infection period and taken to the laboratory for metabolites activities analysis, and organs were harvested for the determination of organosomatic indices, to determine the potency of the experimental diets as prophylatics, and the results were compared to the one obtained in the blue crown feed (known commercial feed).
- Blood Extraction
The fish was blind folded by covering the head with a thick cloth to attain calmness and blood was extracted via kidney puncture through the genital opening using 5ml injection syringe.
- Metabolite Analysis
Spectrophotometric analysis was performed on the blood plasma using a spectrophotometer model “SURGISPEC SM – 230” manufactured by surgifield medical in England and was used according to manufacturers instruction.
The following Metabolites: total protein (TP); urea (UR); creatinne (CR); albumin (ALB); globulin (GLB) and albumin-globulin ratio (A/G) were determined according to their required wave lengths and reagents as follows:
- The spectrophotometer was adjusted through a control till the reading on the screen shows zero (o) transmittance.
- A blank solution was prepared with the reagents except the samples it was put into a cuvette and insert into the sample compartment of the spectrophotometer.
- The spectrophotometer was set to 100% transmittance using the absorbent control and the cuvette with the blank solution was removed.
- The sample cuvette was wiped clean and insert into the sample compartment of the spectrophotometer, and its absorbance and transmitting value was on the screen.
- The sample curvette was removed and the transmittance reading went back to zero.
- The process were repeated for all the sample adjusting wave lengths accordingly and using the recommended reagents to determine the required parameters.
- The metabolites were calculated using the formalar
X = AT/AS X concentration of standard
Where X = Calculated parameter
AT = Absorbance of test
AS = Absorbance of standard.
- Organosomatic Indices (OSI)
Organs such as liver, spleen, heart, visceral organs, gonads, gill and interperetoneal fats were harvested from the various treatments at the end of the one week post infection period and their organosomatic indices (OSI) were calculated as follows:
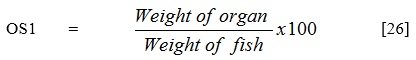
Data analysis
SPSS statistic software 17.0 for windows was used for the analysis, followed by one way analysis of variance to determine deviation in variables among treatments. Comparison among treatments was done using Turkey multiple comparison [27].
Results
Organosomatic indices of Clarias gariepinus fed with experimental diets and exposed to S. aureus
The Organosomatic indices: hepatosomatic index (HSI); viserosomatic index (VSI); gonadosomatic index (GSI); Gilosomatic index (GI-SI); interperotoneal fat (IF); cistosomatic index (CSI) and spleenosomatic index (SSI) of Clarias gariepinus fed the experimental diets for eight (8) weeks and infected with S. aureus for seven days are presented in (Table 1).The results indicates that after infection, there was no significant difference in HSI across the groups. There was significant difference in VSI, significantly lower value was recorded in fish fed Do (3.54 +2.43) and BC (3.81+ 4.95) while fish fed D1-D3 were high and similar in values, but fish fed D4 had significantly higher 5.72+5.33 value. The SSI and CSI were similar across the diets. The value for the GSI was significantly lower in Do fed fish 0.20+0.20 and BC (0.99+0.85) followed by D1 (1.89+0.59), D2 (2.72+0.06) and D3 (2.49+1.78), but was significantly higher in D4 (3.03+3.71). The GI-SI was significantly higher in fish fed Do (2.08 + 1.04) and BC (2.05 + 1.40), but significantly lower and similar in fish fed D1 – D4, while IF was low across the diets but significantly higher in D1 (1.97+0.47), and BC (1.64 +0.37).
|
Experimental Diets (ml/Kg) |
HSI |
VSI |
SSI |
CSI |
GSI |
GI-SI |
IF |
|
|
|
|
|
|
|
|
|
|
Do |
0.75±0.9a |
3.54±2.43a |
0.07±0.07a |
0.08±0.05a |
0.20±0.20a |
2.08±1.04b |
1.97±0.47b |
|
D1 |
0.80±0.9a |
4.60±2.14a |
0.09±0.03a |
0.09±0.04a |
1.89±0.59b |
1.87±1.75 a |
0.69±0.37 a |
|
D2 |
0.85±0.61a |
4.18±0.96a |
0.09±0.05a |
0.07±0.05a |
2.72±0.06c |
1.56±1.96 a |
0.48±0.18 a |
|
D3 |
0.99±0.22a |
4.63±2.18a |
0.09±0.08a |
0.07±0.50a |
2.49±1.78c |
1.49±2.04 a |
0.56±0.38 a |
|
D4 |
0.80±0.57a |
5.72±5.33b |
0.09±0.06a |
0.09±0.53a |
3.03±3.71d |
1.63±1.27 a |
0.42±0.24 a |
|
BC |
0.94±0.66a |
3.81±4.95a |
0.09±0.03a |
0.08±0.57a |
0.99±0.85a |
2.05±1.40 b |
1.64±0.37b |
Table 1: Organosomatic indices of the experimental fish fed dietary S.malaccence for eight (8) weeks and infected with S. aureus for seven (7) days.
Means within the same column with different superscripts are significantly different (P<0.05)
Key: HSI – Hepatosomatic index, VSI – Viscerosomatic index, GSI – Gonadosomatic index, GI – SI – Gilotosomatic index, IF – Interperetoneal fat, CSI – Cistosomatic index SSI – Spleenosomatic index
- Metabolites activities in the plasma of the experimental fish after four (4) and eight (8) weeks of feeding with the experimental diets
The Metabolites activities in the plasma of Clarias gariepinus after four (4) and eight (8) weeks of feeding with the experimental diets are shown in (Tables 2&3) respectively.
|
Experimental Diets |
T.P(g/l) |
UR (muol/L) |
CR (muol/L) |
ALB(g/l) |
GLB(g/l) |
A/G |
|
Do |
46.33±3.21a |
1.70±0.20 a |
42.00±7.00 b |
25.68±2.08 a |
20.67±1.15 a |
1.24±0.12 a |
|
D1 |
47.33±1.53 a |
1.67±0.25 a |
39.00±6.56 a |
28.00±2.89 a |
19.33±2.89 a |
1.45±0.35 a |
|
D2 |
48.67±8.50 a |
2.00±0.36 b |
43.33±9.72 b |
29.00±7.02 a |
19.67±2.31 a |
1.47±0.23 a |
|
D3 |
48.33±4.72 a |
1.83±0.35 a |
46.33±7.57 b |
28.00±0.58a |
20.33±0.58 a |
1.37±0.17 a |
|
D4 |
47.00±9.44 a |
1.57±0.12 a |
40.33±8.74 b |
26.13±4.73 a |
20.67±4.73 b |
1.27±0.12 a |
|
BC |
49.00±9.54 a |
1.83±0.12 a |
44.33±8.33 b |
30.33±2.08 a |
18.67±2.08 a |
1.62±0.1 a |
Table 2 : Metabolites Activities in Plasma Biochemistry of the experimental fish after four (4) weeks of feeding with dietary S.malaccence.
Means within the same column with different superscripts are significantly different (P < 0.05)
Key:T.P – Total protein, UR – Urea, CR – Creatinine, ALB – Albumin, GLB – Globulin, A/G–Albumin Glabulin
|
Experimental Diets |
T.P(g/l) |
UR (muol/L) |
CR (muol/L) |
ALB(g/l) |
GLB(g/l) |
A/G |
|
Do |
49.67±3.06a |
1.60±0.30 b |
50.67±10.06 b |
27.67±1.53 a |
22.00±1.00 a |
1.26±0.06 a |
|
D1 |
45.33±2.52 a |
1.47±0.21 a |
44.00±14.42 a |
25.33±2.31 a |
20.00±2.00 a |
1.27±0.06 a |
|
D2 |
51.0h0±3.60 b |
1.40±0.17 a |
44.67±17.79 a |
30.00±4.51 a |
21.00±0.00 a |
1.21±0.15 a |
|
D3 |
49.00±3.60 a |
1.07±0.12 a |
42.67±8.62 a |
26.33±2.63 a |
22.67±2.52 a |
1.16±0.21 a |
|
D4 |
52.33±3.06 b |
1.60±0.46 a |
43.67±6.51 a |
30.08±4.04 a |
22.33±1.15 a |
1.34±0.12 a |
|
BC |
50.33±6.81 b |
2.07±0.71 b |
51.67±7.23 b |
28.66±2.65 a |
21.67±4.72 a |
0.91±0.12 a |
Table 3: Metabolites Activities in Plasma Biochemistry of the experimental fish after Eight (8) weeks of feeding with dietary S.malaccence.
Means within the same column with different superscripts are significantly different (P < 0.05)
Key:T.P – Total protein, UR – Urea, CR – Creatinine, ALB – Albumin, GLB – Globulin, A/G–Albumin Glabulin
After four (4) weeks of feeding there was no significant difference in TP, the values were within the same range (46.33+3.21 – 49.00 +9.54) across the diets. The value for UR in fish fed D2 was higher (2.00+0.36) while the rest were within the same range (1.67+0.25 – 1.83 + 0.12), CR was significantly lower in the fish fed D1 (39.00+6.56) while the rest were higher and within the same range (40.33+44.33+8.33). There was no significant difference in the ALB, it was similar across the diets (25.68 +2.08 +30.33 +2.08), the GLB was significantly higher in fish fed Do (20.67+1.15), D3 (20.33+0.58), and D4 (20.67+4.73), while fish fed D1, D2 and BC were lower and within the same range, there was no significant difference in the A/G ratio across the experimental diets.
After eight (8) weeks of feeding, the value for the TP was significantly lower in fish fed Do, D1 and D3 but significantly higher in fish fed BC (50.33+6.81), D2 (51.00+3.60), and D4 (52.33+3.06). The UR was significantly higher in fish fed BC (2.07+0.71), while fish fed Do – D4 were lower and similar 1.07+ 0.12+1.60+0.46. The CR was significantly higher in fish fed BC (51.67+7.23) and Do (50.67 + 10.06), but similar in the rest diets. In ALB there were no significant difference across the experimental diets (25.35+2.31 – 30.08+4.04) also there was no significant difference in GLB (21.00+0.00 – 22.67+2.52), and in A/G there were no significant difference they were within the same range in Do – D4 (1.21+0.55 – 1.34+0.12, but was significantly lower in fish fed BC (0.91+0.12).
- Metabolites activities in plasma of experimental fish after eight (8) weeks of feeding with dietary S. malaccence and infected with S. aureus
The metabolites activities in the plasma of Clarias gariepinus after eight (8) weeks of feeding with dietary S. malaccence and infected with S. aureus are shown in (Table 4). There were no significant difference in the TP across diets (40.33+1.15 – 43.33+1.53), meanwhile there were significant difference in the values of UR, fish fed Do and BC had significantly higher values 2.20+0.46 and 2.23+0.42 respectively, while fish fed D1 – D4 were significantly lower but similar. The values of CR were significantly high in the fish fed Do (53.33+10.21) and (54.67+5.51), but were similar in fish fed D1 – D4 (41.67+18.93 – 47.00+14.73).
The values of ALB were significantly lower in the fish fed Do and BC, 16.33+0.53 and 16.33 +0.53 respectively, while fish fed D1 – D4 were higher and similar. The value for GLB was significantly higher in fish fed BC, but similar in fish fed Do – D4. The values for AG were higher in fish fed D2 and D4 compared to the rest, but were in the same range.
|
Experimental Diets |
T.P(g/l) |
UR (muol/L) |
CR (muol/L) |
ALB(g/l) |
GLB(g/l) |
A/G |
|
Do |
40.33±1.15a |
2.20±0.46 b |
53.33±10.21 b |
16.33±0.58 a |
24.67±0.58 a |
0 0.66±0.03 a |
|
D1 |
47.33±4.73 a |
1.43±0.32 a |
47.00±14.73a |
23.33±3.06 b |
24.00±0.58 a |
0.97±0.15 a |
|
D2 |
47.67±4.51 a |
1.37±0.15 a |
45.33±18.93 a |
25.33±5.51 b |
22.34±5.51 a |
1.13±0.23 b |
|
D3 |
48.33±1.53 a |
1.23±1.12 a |
42.67±10.79 a |
23.67±1.53 b |
24.66±1.15 a |
0.96±0.16 a |
|
D4 |
48.00±8.00 a |
1.87±0.87 a |
41.67±18.93 a |
24.00±4.00 b |
24.00±4.04 a |
1.00±0.11 a |
|
BC |
45.67±4.93 a |
2.23±0.42 b |
54.67±5.51 b |
16.33±1.53 a |
29.94±2.00 a |
0.86±0.02 a |
Table 4: Metabolites Activities in the Plasma of the experimental fish after Eight weeks feeding with dietary S.malaccence and infected with S. aureus.
Means within the same column with different superscripts are significantly different (P < 0.05)
Key: T.P – Total protein, UR – Urea, CR – Creatinine, ALB – Albumin, GLB – Globulin, A/G–Albumin Glabulin
Discussion
- Organosomatic indices of C. gariepinus fed the experimental diets and infected with S. aureus
The Organosomatic indices are often used to evaluate the health status of fish [28,29]. In this research, there were no significant difference in the HSI, SSI and CSI, after the period of infection with the experimental pathogen. This result is similar to the one of Ukwe and Jamabo [25] who reported no significant difference in the HSI, SSI and CSI when C. gariepinus was fed dietary Mangnifera indica back extract and exposed to P.aeruginosa. Gupta et al, [30] also reported no significant difference in the CSI and SSI when Rattus rattus was parasitized. This result could be attributed to the fact that the experimental pathogen was not virulence on the liver, spleen and heart of the experimental fish. The VSI was higher in the fish fed D4 followed by the fish fed D1, D2 and D3, this could be as a result of the enhance health in the fish organs, probably as a result of the phytochemicals presence in S. malaccence, which are not only antibacterial against the pathogen, but also growth promoters [31,32], but it was lower in the fish fed Do and BC. The interperetoneal fat was significantly higher in the fish fed Do and BC, and significantly lower and similar in the fish fed D1-D4. The result of the interperetoneal fat is in agreement with the report of Ukwe and Entire [28] who reported increase in interperetoneal fat (visceral fat) when C. gariepinus was exposed to P. aeruginosa. The increase in the interperetoneal fat in the fish fed Do and BC could be as a result of innate immune response of the experimental fish against the experimental pathogen, this argument is supported by Jenab et al [33] and Ukwe and Etire [28]. The absence or reduction of the interperetoneal fat in the fish fed D1-D4 and exposed to the pathogen could be due to the presence of the antimicrobial phytochemicals in S. malaccence that have prevented the virulence of the S. aureus against the fish [34]. Contrary to the interperetoneal fat, the gonadosomatic index (GSI) was significantly lower in the fish fed Do and BC, and significantly higher in the fish fed D1-D4. This could be as a result of the presence of the excess fat in the abdominal cavity of the fish fed Do and BC, as a result of the presence of the pathogen (S. aureus), since increase in interperetoneal fat has been observed as an innate immune response in fish [28,33].
- Effect of the Experimental diets on the Metabolites of C. gariepinus
At the end of the four weeks feeding with the experimental diets Do-D4, and BC, there was no significant difference in the analysed metabolites apart from Creatinine (CR). All the metabolites plays a unique role in the metabolism of the fish. The total protein (TP) indicates the state of the fish innate immune response [22], the albumin (ALB) and globulin (GLB) are associated with improved non-specific immune response, and plays a role as plasma protein carriers [35],the urea (UR) is a major product of the breakdown of complex compound (protein) in the body [36], while Creatinine, a major metabolites in the body is said to be a good assessor for the proper functioning of the kidney [37]. However, after eight (8) weeks feeding there was still no significant difference in the UR, CR, ALB, GLB and A/G in the fish fed D1-D4 indicating that the administered diets did not alter the composition of these metabolites in the fish plasma. But there were significant increase in the TP of the fish fed D2, D4 and BC. This results is similar to the observations of Binali et al [38] when Huso huso juveniles were fed dietary Urtica dioica. This result depicts that the dietary S. malaccence at the administered dose has the capacity to improve innate immune response in C. gariepinus [22,39]. However the value of the UR and CR in the fish fed Do and BC increased. The increase in UR and CR in fish plasma indicates the inability of the fish kidney to discharge them [40]. The implication of this is that the dietary S. malaccence at the used concentrations has the capacity to maintain the functionality of the kidney, even at prolong usage.
- Prophylactic effect of the Experimental diets on the Metabolites of S. aureus infected C. gariepinus
After seven (7) days post infection the TP was significantly lower in the fish fed Do and BC, and increased significantly in the fish fed D1 – D4. This result is similar to the report of Mohamad and Abasali [41] who reported the effect of A. hydrophila on common carp fed plants extracts supplemented diets, and Dos Santos et al, [42], who reported effects of A. hydrophila on silver catfish fed supplemented A.triphyla diets. The improved TP in the fish fed D1-D4 depicts non-specific immune response of the fish [35] arising from the phytochemicals contained in the S. malaccence diets, resulting to improve energy due to high protein metabolism [43]. There was significant increase in the UR and CR in the plasma of fish fed Do and BC after seven (7) days post infection period. Increase in UR and CR have been associated with kidney malfunction [44], and Murray et al [45] attributed increase UR in fish plasma as gill dysfunction and this could be as a result of the pathogen infection [40], and can lead to alterations in the production of white blood cells, since white blood cells are produced in the kidney [46]. The observation of Murray et al [45] could be the reason why there was inflammation (increase in size) in the GI – SI of the infected fish fed Do and BC compared to the fish fed D1-D4. The uniform GI-SI, UR and CR presence in the plasma of the fish fed D1-D4 could be as a result of the presence of the phytochemicals contained in S. malaccence that are believe to be bactericidal to the experimental pathogen [34]. ALB was significantly lower in fish fed Do and BC, but increase significantly in the fish fed D1-D4, this result is similar to the result of Abasali [41] who reported the effect of A. hydrophila on common carp fed plants extract supplemented diets, and Dos Santos et al, [42] who reported the effects of A. hydrophila on silver catfish fed supplemented A. triphyla diets. The decrease in ALB can be attributed to the virulence of the pathogen (S. aqueous) on the C. gariepinus. Charlie-Silva et al [47] reported decrease in ALB as a way of responding to the negative effect of bacterial presence in fish, when Tilapia was innoculated with A. hydrophila. Phytochemcials such as the ones present in S. malaccence have been reported to be bactericital and bactercostatic to pathogens such as S. aureus [34]. The higher value of ALB in the C. gariepinus fed D1 – D4 after infection could be as a result of the presence of the phytochemcials in the diets which may have hindered the virulence of the S. aureus on the C. gariepinus [9,25]. Though values of GLB was higher after infection in all the treatments compared to their values before infections, it was significantly higher in the fish fed BC. The increase in GLB in fish plasma have been associated with the presence of bacteria in fish [47].
Conclusion
The present results indicates that there was no significant difference in the HSI, SSI and CSI of the experimental fish after seven (7) days post infection, this can be attributed to the fact that the experimental pathogen was not virulence on the liver, spleen and heart of the experimental fish. The presence of interperetoneal fat was significantly higher in the fish fed Do and BC compared to the fish fed D1-D4 and this can be attributed to the virulence of the pathogen on the fish fed Do and BC. It can be concluded that the presence of disease or pathogen such as S. aureus in fish especially catfish could lead to increase in interperetoneal fat, with the resultant effect of reducing the fish gonads, indicating negative effect on the fish reproductive capacity. The presence of the pathogen also causes increase in Urea, Globulin and Creatinine in fish, which is an indication of infringement on the fish kidney and gills, and this can alter the production of red blood cells and the fish respiratory system. The increase in GLB and the reduction in ALB was also observed as one of the consequence of the presence of the bacteria (S. aureus). The phytochemicals present in S. malaccence posses antimicrobial effects that are either bactericidal or bactereastatic to the tested pathogen (S. aureus), as observed in the fish fed D1-D4. S. malaccence at the used concentrations can be used to prepare medicinal diets for fish especially C. gariepinus as a prophylactic.
References
- Shim SM, Ferruzzi MG, Kim YC, Janke FM Santerre CB (2009) Impact of phytochemical rich foods on bioaccessiblity of mercury from fish. Food Chemistry 112: 46-50.
- James LA, Frank A, Taryn G (2019) Economics of aquaculture policy and regulation. Annual Review of Resource Economics 11: 101-123.
- FAO (2007) The state of World Fisheries and Aquaculture: 2006 FAO Fisheries and Aquaculture Deparment, Rome.
- Eric CO, Kingsley IO, Ertan A (2018) The economic viability of utilization of biogass as an alternative source of energy in rural parts in Nigeria. International Journal of Global Energy Issues 41: 205-225.
- Villa-cruz V, Davila J, Viana MT, Vazquez-Dulialt R (2009) Effect of bronccoli (Brassica oleracea) and its phytochemcial sulforaphane in balance diets on the detoxification enzymes levels of tilapia (orechronics niloticus) exposed to a carcinogemic and mutagemic pollutant. Chemosphere 74: 1145-1151.
- Rico A, Phu TM, Satapomvanti K, Min I, Shahabudin AM, et al. (2013) Use of veterinary medicines feed addivities and probiotics in four major international traded aquaculture species farmed in Asia. Aquaculture 412-413.
- Ringo E, Louino L, Kristianen M, Salinas I, Myklebust R, et al. (2010) Lactic acid bacteria vs pathogens in the gastrointertinal tract of fish. A review. Aquactulture Research 211: 1365-2019.
- Ukwe IOK, Gabriel UU (2019) Herbs and Herbal supplements: key to a productive, Healthy and Eco-friendly aquaculture. Delta Agriculturist 11: 55-67.
- Ukwe IOK, Edun OM, Akpomejoro J (2022a) Medicinal phytochemicals in Chromotaena odorata and its effect on Clarias gariepinus Infected with Staphylococcus aureus. International Journal of farming and Allied Science, 11: 41-52.
- Aveen NA (2015) Phytochemical analysis and evaluation antibacterial activity of citrus medica peel and juice growing in Kurdistan/Iraq. Journal of Applied Phamacentical Science 5: 136-141.
- Ukwe IOK, Deekae SN (2022b) Phytochemical Assessment of Persea americana powdered leaves and its potency in protecting Clarias gariepinus against Klebsiella pneumonia. Asian Journal of Fisheries and Aquatic Research 16: 1-9.
- Krishnaiah D, Sarbathy R, Bono A (2007) Phytochemical antioxidants for health and medicine a move towards nature. Biotechnology molecular Biological Review 2: 97-104.
- Chakrabordy SB, Horn P, Hancz C (2013) Application of phytochemicals as growth-promoters and endocrine modulators in fish culture. Review in Aquaculture 5: 1-9.
- Idowu TA, Anthony P (2022) Occurrence of Parasites in Clarias gariepinus upper River Benue Valley Area, Yola, Adamawa State. Book of Abstracts of the 37th Annual Conference of Fisheries Societies of Nigeria (FISON) 15.
- Thora L, Thomas M, Seyed HH, Bo P, David LS, et al. (2020) Sustainable aquaculture requires environmental-friendly treatment strategies for fish disease. Reviews in Aquaculture 12: 943-965.
- Shamsi S (2019) Seafood-born parasitic diseases: A “one health” approach is needed. Fishes 4: 9.
- Ukwe IOK, Vopnu FB (2021) Disease Resistance and Enzymatic changes in Pseudomonas aeruginosa Infected Clarias gariepinus treated with Carica papaya root extracts. Journal of medical care Research and Review 2589-8949.
- Ukwe IOK, Oladapo-Akinfolarin TT (2015) Alternation in Enzyme Activities of Clarias garipienus infected with Aeromorios hydrophila and Pseudomonas aeruginosa. Asian Journal of Fisheries and Aquatic Research 4: 1-9.
- Adeyemi JA (2014) Oxidative stress and antioxidant enzymes activities in African Catfish Clarias gariepinus experimentally challenged with Eshenchina Collaord vibro Fish physiology and Biochemistry 40: 347-354.
- Ukwe IOK, Abu OMG (2021) Effect of Dietary Persea Americana on the organosomatic indices, Disease Resistance and Liver Histopathology of Clarias gariepinus Expose to Klebsiella pneumonia. Asian Journal of Fisheries and Aquatic Research 15: 148-156.
- Bahmani M, Kazemi R, Donskaya P (2001) A comparative study of some heamatological features in young reared sturgeon (Acipenserpersicus and Husa huso). Fish physiology and Biochemistry 24: 135-140.
- Awad E, Austin D, Lyndon AR (2013) Effect of black cumin seed oil (Nigella Satiua) and nettle extract (Gueicetin|) on enhancement of immunity in rainbow trout. Ohcorhynchus mykiss (Walbaum) Aquaculture 388/391: 193-197.
- Pulliat T (2006) Encyclopedia of world medicinal plants, Regency publications 1082.
- Savitha RC, Padmavathy S, ASundhararajan (2011) In vitro antioxidant activities on leaf extracts of syzygium malaccence (L) Merr and perry. Ancient science of life 30: 110-113.
- Ukwe OIK, Jamabo NA (2020) Effect of dietary mango bark (MangniferaIndica) extract on Clarias gariepinus (Burchell, 1822) infected with Pseudomonas aeruginosa. World Journal of Fish and Marine Sciences 12: 74-80.
- Dekic R, Savie N, Monojlovic M, Golub D, Pavlicevic I (2016) Condition factor and organosomatic indices of rainbow trout from different brood stock. Brotechnology Animal Husbandary 32: 229-237.
- Wahua TAT (1999) Applied statistics for scientific studies. Africa links books. Aba Nigeria 365.
- Ukwe IOK, Etire DI (2021) Effects of Persea americana leaves on the Enzymes and Organosomatic indices of Pseudomonas aerugionosa Infected Clarias gariepinus. Journal of Medical Care Research and Review 4: 1-29.
- Ronald WG, Bruce AB (1990) Organosomatic indices and antopsy base assessment as an indicators of health condition of fish. Journal of American fisheries society 8: 98-108.
- Gupta N, Gupta DK, Sharma PK (2016) Condition factor and organosomatic indices of parasitized Ratus rattus as indications of host health. Journal of Parasistic Diseases 61:21-25.
- Emriadi P, Untair, Efdi M (2021) Leave extract of Syzygium malaccense as green inhibitor of mild steel in acidic medium. Rasayan Journal of Chemistry 14: 569-577.
- Chakrabordy SB, Heniz C (2011) Application of Phytochemicals as Immunostimulant, anti-pathogenic and anti-stress agents in finfish culture. Reviews in Aquaculture 3: 103-119.
- Jenab A, Roghanian R, Emtiai G (2020) Bacterial Natural Compounds with Anti-Inflammatory and Immunomodulatory properties (Mini-Review). Drg Design Development and theraphy 4: 1-15.
- Quenon C, Hennebelle T, Butaid JF, Ho B, Samailie J, et al. (2022) Antimicrobial Properties of Compound Isolated from Syzygium Malaccence (L) Merr. and L. M. Perry and Medicinal Plants used in French Polynesia. National Library of Medicine 12: 733.
- Sivagurunathan A, Innocent BX, Sarasuathi SG, Mariappan A (2012) Immunostimulatory Potential Dietary Amila (Phyllanthus emblica) in Growth and Haematology of Tilapia Mossambiicuss challenged with Psecudomonas acruginosa. International Research Journal of Pharmacy 3: 165-168.
- Adam TE, Ellis C, Jones SH (2017) Autoethnography. In the International Encyclopedia of communication Research Methods (pp.1-11) Hobaken, N. J. John Wiley & Sons
- National Kidney Foundation (2002) Clinical practice guidelines for Chronic kidney diseases. evaluations, classification and stratification. America Journal of Kidney Diseases 39: 1-266.
- Binali M, Ghasi M, Farabi SMV, Ourgholam R, FAli H, et al. (2014) Immunological Parameters in Juvenile beluga (Huso huso) following the diet supplemented with nttle (Nrtica doila). Fish Shellfish Immunology 36: 46-51.
- SahuS, Das BK, Mishra BK, Pradhan J, Sarangi N (2007) Effect of Allium Statium on the Immunity and Survival of Labeo rohita Infected with Aeromonas hydrophila. Journal of Applied Ichthyology 1: 80-86.
- Mahmoud AE, Mona SZ, Abdel RY, Dsouky HA, Osman KAH, et al. (2011) Study on clinopathological and biochemical changes in some freshwater fishes infected with external parasites and subjected to heavy metals pollution in Egyptian. Life science Journal 8: 401-405.
- Mohamad S, Abasali H (2010) Effect of plant extracts supplemented diets on immunity and resistance to Aeromonus hydrophila in common carp (Cyprimis carpio). Agricultural Journal 5: 119-127.
- Dos-Santos AC, Sutili FJ, Heinzmann BM, Cunha MA, Brusque ICM, Baldisserotto B et al. (2017) Alosia triphyla essential oil as additive in silver catfish diet: Blood response and resistance against Acromonas hydrophila Infection. Fish and Shellfish Immunology 62: 213-216.
- Adamu KM, Kori SO (2011) Effect of Subtlethal Concentrations of tabacco (Nicotiana tobacum) leaf dust on esome biochemical parameters of hybrid catfish (Clarias gariepinus and Heteabranchus bidorsal). Brailian archieve of Biology and Technology 54: 183-193.
- Nnubuchi UO, Ejikeme OG, Didiugwu NC, Ncha OS, Onahs, SP et al. (2015) Effect of parasites on the biochemical and haematological indices of some clarid (silumformes) catfish from Anambra Rivers, Nigeria. International Journal of Fisheries and Aquatic Studies 3: 331-336.
- Murray RKD, Rranne PAM, Roswell VW (1990) Harper's Biochemistry. Norwalk Connecticut Los Altos, California.
- Kumar V, Mandal SC, Baman D (2011) White Blood cells and its formation in fish immune system. Aqua International 25: 1-4.
- Charle-Silva I, Klein A, Gome JMM, Prado ER, Moraea SFE, et al. (2010) Acute-phase proteins during inflammatory reaction by bacterial infection: Fish model. Scientific Report 1-13.
Citation: Ukwe IOK, Abu OMG and David GJ (2023) Prophylatic Potentials of Dietary Syzgium malaccence leaves Extracts on the Metabolite Activities and Organosomatic Indices of Clarias gariepinus. J Aquac Fisheries 7: 64.
Copyright: © 2023 Ukwe IOK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

