
Some Characteristics of Reproduction in the Natural Environment of the African Giant Frog (Conroua Goliath) (Boulenger, 1906) In the Monomodal Forest Zone of Cameroon
*Corresponding Author(s):
Nguessu CharlesLaboratory Des Ressources Aquatiques, Département De L’Aquaculture, Institute Des Sciences Halieutiques, Université De Douala. BP 7236-Douala, Cameroon
Tel:(+237) 677693018/65698067,
Email:cnguessu2011@yahoo.fr ; infos.ish@univ-douala.com
Abstract
During 2 breeding seasons (2021-2022), 44 C. goliath nests were inventoried in 3 rivers (GOUNDJA, BETE, TIGUE) with the help of fishermen/hunters. This study showed that C. goliath breed exclusively in the dry season (November to April). The distribution of C. goliath nests was significantly different according to breeding period and site (p < 0.05). Depending on the site, the higher mean frequency was inversely proportional to human activity. C goliath nests are rectangular quadrilaterals. The average surface areas calculated were between 0.30 ± 0.17 m2 and 0.13 ± 0.05 m2. The average nest height is 5 ± 1cm. The colour of the eggs varied from whitish on day 1, greyish on day 2 and black on days 3-4-5, and the shape from round on days 1 and 2 to oval on days 3-4-5. The average diameter of the eggs was 6.92 ± 0.25 mm. The average weight of the larvae at hatching was 90 ± 0.5mg and the average size of the larvae at hatching was 6.92 ± 0.25mm. 95% of the nests were on parental control. Physico-chemical parameters varied very little (pH, to, conductivity, dissolved oxygen) with respective averages of 6.60 ± 0.12; 28.31 ± 0.24oC; 44.04 ± 0.11µS/cm, 18.13 ± 0.15mg/l. Average relative fecundity was 11974 ± 7061 eggs/kg of clutch. The average fertility rate was 99.49 ± 0.22%. The average hatching rate was 98.47 ± 1.87% in nests under parental control and 92.35 ± 30% in nests under parental control.
Keywords
Conroua goliath, Incubation, nest, parental control, orphan nest.
Introduction
Biodiversity is of particular importance because it has a value beyond the needs and requirements of human beings and independent of their existence that is qualified as non-anthropocentric values [1-3]. Today, the erosion of biodiversity is a major problem. According to the IUCN, 25% of the world's mammal species and 11% of birds are directly threatened with extinction. As for the other, less well-known biological groups, scientists predict that they will be 25-50% extinct by the end of the century if no appropriate measures are taken. This is why we speak of the 6th extinction caused by the activities and development of the human species, in reference to the 5 major extinctions due to natural causes (fishing, pollution and eutrophication, the physical degradation of habitats, the invasion of exotic species and climate change) that have previously marked the history of life on Earth [4]. Recent investigations report an alarming decline in populations of frogs and other amphibians worldwide [5-9] The goliath frog (Conraua goliath) is the largest frog in the world, measuring up to 300 mm and weighing over 3 kg. This species is endemic to Cameroon, Equatorial Guinea and possibly northern Gabon (Boulenger, 1906). The goliath frog is classified as an endangered species (IUCN, 2004). Knowing the parameters of reproduction and protecting the breeding area is an existential condition for any living being, regardless of its resilience. One of the conservation measures for this species would be in situ protection of its breeding environment. The aim of this study is to contribute to improving our knowledge of the reproduction of goliath frogs in the wild. More specifically, depending on the study period and sites, the aim will be to evaluate the frequency of egg-laying per site and per period, the characteristics of the nests (shape, size), the characteristics of the eggs (color, average weight, diameter, incubation time, to determine the fecundity, fecundity rate and hatching rate), and to characterize the larvae at hatching (average weight and average size at hatching and the rate of deformed larvae) per site.
Materials And Methods
Ethical statement
This study was approved by the Ministry of Forests and Fauna (MINFOF) under reference
5188/L/MINFOF/SG/DFAP/SDCF/SEP/ESH and covered the entire study area.
The animals used in this study were not sacrificed and all were released alive into their environment of origin after use.
Study area
The study was carried out between November and April 2021 and 2022 in sub-Sahelian Africa, more precisely in the Littoral Region of Cameroon, and made it possible to characterise the reproduction of Conroua goliath in 03 rivers in the Loum district (Goundja, Bete and Tigue) located respectively at the following positions (Figure 1) (LN : 04,39596o ; LE :12,92584o), (LN : 04,64054o ; L E : 009,75200o), (LN :04,72304o ; LE 009,984179o) [10].
 Figure 1: Location of the study area.
Figure 1: Location of the study area.
Relief, Soil, Climate and Hydrography
The study area is dominated by 2 types of relief: the plateau and the hills, which are strewn with watercourses with steep slopes along and across them that are highly vulnerable to erosion. The rivers are lined with volcanic and basaltic rocks. The riverbeds are dominated by sandy-loam soil. The climate is equatorial. This climate is strongly influenced by the monsoon and is characterized by a short dry season (November to March) and a long rainy season (April to October). The average rainfall is 2,700 mm, the average temperature is 26°C and relative humidity is 75%. The hydrography is dense and is crossed by the BETE, GOUNDJA, TRU WATER, MABOMBE, TIGUE and SATIE rivers.
During the study period (March to November 2021 and 2022), goliath frog nests were inventoried in 3 MOUNGO watercourses (GOUNDJA, BETE, TIGUE). The number of nests per site and per breeding period was recorded and characterized. The principle of the Kilometric Abundance Index was applied in the process [11-15]. The Kilometric Index of Abundance (KIA) is a method for measuring the relative abundance of species along a route. It was developed in 1958 by Ferry and Frochot1 and, in a homogeneous environment, makes it possible to obtain an abundance per kilometre for each species. For each watercourse, whenever C. goliath nests were observed, the shape and dimensions were recorded using a tape measure, i.e. the height of water in the nest (depth), the length of the nest, the width of the nest or the diameter of the nest, and these values were used to calculate the average nest surface area (ST). Similarly, the speed of water circulation in the nest was measured by placing a float at point A at the entrance to the nest and timing the time taken to leave the nest at point B. A stopwatch was used to determine this time. Thus, d being the distance travelled by the float from point A to point B and t the time taken to cover this distance, the speed of water circulation in the nest is given by the formula
V=d/t
The number of eggs laid, the number of clear eggs, the number of fertilized eggs and the number of dead eggs were determined using the Practical Method for Estimating Seed Numbers developed by Arvalis in 2003 with the use of plots. This method made it possible to calculate the fecundity, fecundity rate, hatching rate and deformed larva rate.
Characteristics Studied
Fecundity
The fecundity of goliath frogs was determined by the formula
F= number of eggs/kg of eggs laid
Fertilization rate
For each nest observed, the number of eggs was estimated by counting them. A square plot with a surface area of 25 cm2 was used to estimate the number of eggs laid. The plot was placed on the surface of the nest and all the eggs contained in the plot were counted. This number is made up of fertilized and unfertilized (clear) eggs. The total number of eggs in the nest is then deducted using the following formula N= (ST/SP) x n
N: Number of eggs laid in a nest (fertilized eggs + unfertilized eggs) ;
ST: Total surface area of nest;
SP: Surface area of plot
n: Number of eggs counted in a plot
The same method is used to determine the number of unfertilised eggs (light-coloured eggs).
N‘= (ST/SP) x n’
n': number of clear eggs in the plot
N': number of clear eggs contained in a nest
The fertilization rate is T fertilization= (N-N') x100/N
Hatching rate
To estimate the hatching rate in a nest, we counted the eggs that aborted during incubation using a 25 cm2 plot and deducted the total number of eggs that died during incubation (N eggs that died during incubation) from the surface area of the nest. This same method was used to determine the number of eggs that hatched.
Hatching rate= N hatched x 100/ N fertilised
Rate of deformed larvae:
Egg characteristics
The color, shape, diameter and weight of the eggs were recorded during incubation and the fecundity determined (number of eggs/kg of eggs laid).
Egg color was observed and recorded during incubation;
Egg shape was observed and recorded during incubation;
Egg diameter was measured during incubation using graph paper;
The weight of the eggs was measured using a 0.01g precision electronic balance;
Incubation time was observed;
Larval characteristics
At hatching,
The larvae were characterised
The average weight of the larvae was determined using a 0.01g precision electronic balance;
The average total length of the larvae was measured using graph paper, as was the length of the tail;
The number of deformed larvae was estimated. To estimate the number of deformed larvae in a nest, we counted the deformed larvae using a 25 cm2 plot. The number of deformed larvae was counted in the plot and the total number was deducted from the total surface area.
Number of deformed larvae = (ST/SP) x number of deformed larvae in a plot
-Physicochemical characteristics of nest water
The physicochemical parameters of the nest water (temperature, pH, dissolved oxygen, conductivity and salinity) were measured using a multi-parameter KIT.
Statistical Analysis
The data collected were subjected to analysis of variance (ANOVA). When the difference between the means was significant, Duncan's test was used to separate the means at the 5% significance level. SPSS version 21.0 (Statistical Package for Social Sciences) was used for the analyses.
Results
Physicochemical characteristics of C. goliath egg nests
- Temperature
The monthly change in water temperature in the spawning nests according to watercourse between 2021 and 2022 is illustrated in (Figure 2).
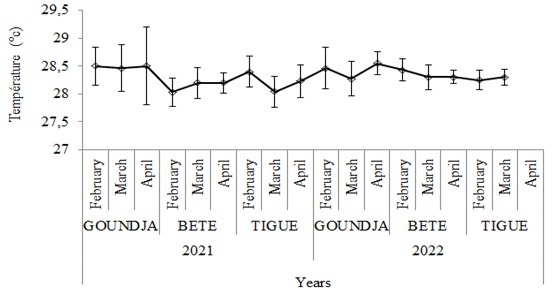 Figure 2: Monthly change in water temperature in C. goliath egg-laying nests.
Figure 2: Monthly change in water temperature in C. goliath egg-laying nests.
nests by river between 2021 and 2022
The water temperature in the egg-laying nests evolved in a jagged pattern at all sites, with the highest values at GOUNJA and BETE in April 2021, and at GOUNDJA and TIGUE in 2022. It was lowest in February at GOUNDJA, and in March at BETE and TIGUE in 2021; however, it was lowest in March at all three sites in 2022. However, no significant difference (p>0.05) was observed between sites and months.
- pH
(Figure 3) illustrates the monthly change in water pH in C. goliath
egg-laying nests by watercourse between 2021 and 2022
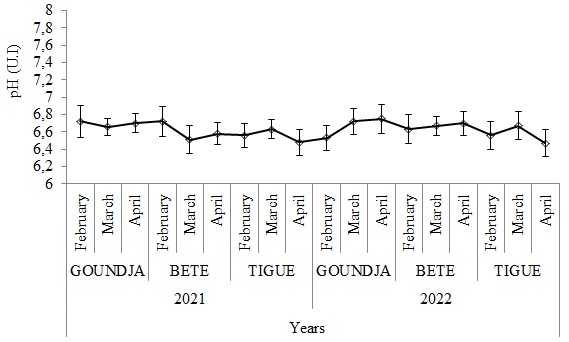 Figure 3: Monthly change in water pH in C. goliath egg-laying nests.
Figure 3: Monthly change in water pH in C. goliath egg-laying nests.
according to watercourse between 2021 / 2022
Like water temperature, pH varied slightly between sites and months.
- Conductivity
The monthly trend in water conductivity in C. goliath egg-laying nests as a function of watercourse between 2021 and 2022 is shown in (Figure 4).
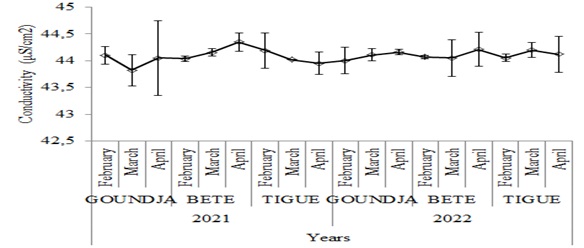 Figure 4: Monthly changes in water conductivity in C. goliath egg-laying nests.
Figure 4: Monthly changes in water conductivity in C. goliath egg-laying nests.
egg-laying nests by watercourse between 2021 and 2022
Conductivity varied little and in a similar way to temperature and pH.
- Dissolved oxygen
(Figure 5) shows monthly changes in dissolved oxygen in C. goliath egg-laying nests by watercourse between 2021 and 2022.
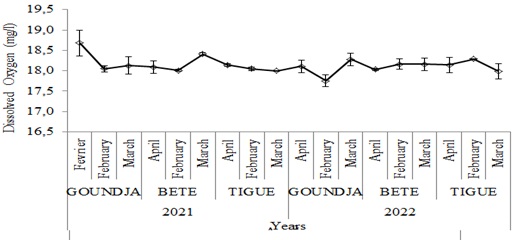 Figure 5: Monthly changes in dissolved oxygen in C. goliath egg-laying nests.
Figure 5: Monthly changes in dissolved oxygen in C. goliath egg-laying nests.
nests by watercourse between 2021 and 2022
Dissolved oxygen varied as saw teeth and the lower values were recorded in March.
- Salinity
Monthly changes in salinity in C. goliath egg-laying nests by watercourse between 2021 and 2022 are shown in (Figure 6).
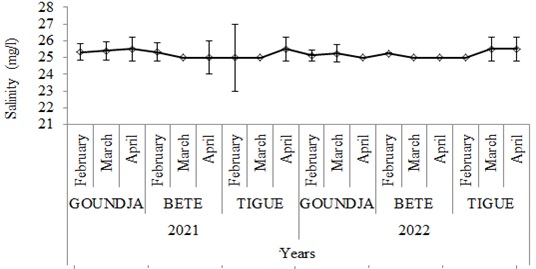 Figure 6: Monthly change in salinity in C. goliath egg-laying nests.
Figure 6: Monthly change in salinity in C. goliath egg-laying nests.
nests by watercourse between 2021 and 2022
The average salinity of the water in the egg-laying nests varied very little between sites and periods.
Characteristics of C. goliath nests
- Overall distribution of C. goliath nests in watercourses
The distribution of egg-laying nests by site is illustrated in (Figure 7).
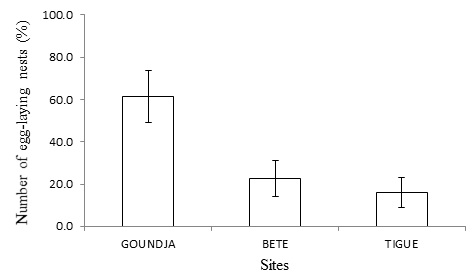 Figure 7: Distribution of C.goliath egg-laying nests (% of total) by site.
Figure 7: Distribution of C.goliath egg-laying nests (% of total) by site.
It can be seen that the number of egg-laying nests was significantly (p < 0.05) higher at the Ngoudja site, with the lowest number recorded at Tigue.
- Monthly distribution of C. goliath egg-laying nests in sites during 2021-2022.
(Figure 8) illustrates the monthly distribution of egg-laying nests by site.
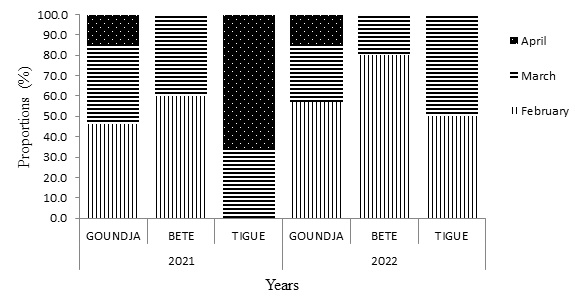 Figure 8: Monthly distribution of C.goliath nests by site during the years 2021/2022.
Figure 8: Monthly distribution of C.goliath nests by site during the years 2021/2022.
Irrespective of the spawning sites, the number of nests was highest in February, followed by March, with the lowest number recorded in April. Taking the sites into account, the number of nests was highest at TIGUE in April.
- Dimensions of egg-laying nests of Conroua goliath
(Table 1) summarises the dimensions of the egg-laying nests and the speed of water flow in the nests in relation to the egg-laying sites.
|
Dimensions of egg-laying nests and water speed |
Sites |
||
|
GOUNDJA |
BETE |
TIGUE |
|
|
Number of egg-laying nests |
27 |
10 |
7 |
|
Water depth (cm) |
5.41 ± 0.73a |
5.50 ± 0.85 a |
5.00 ± 0.28 a |
|
Length of the nest (m) |
0.93 ± 0.42 a |
0.50 ± 0.11 a |
0.93 ± 0.20 a |
|
Wight of the nest (m) |
0.30 ± 0.09 a |
0.26 ± 0.04 a |
0.27 ± 0.08 a |
|
Area of the nest (m2) |
0.28 ± 0.17 a |
0.23 ± 0.05 a |
0.24 ± 0.04 a |
|
Water speed (m/s) |
0.037 ± 0.00 a |
0.04 ± 0.00 a |
0.04 ± 0.00 a |
Table 1: Characteristics of C. goliath egg-laying nests in relation to watercourses.
(a): on the same line, figures with the same letter are not significantly different (P > 0.5).
The depth of water in the nests was greater at BETE and less at TIGUE. The length, width and surface area of the nests were greater at NGOUNDJA and smaller at BETE. Water flow velocity in the nests was higher in BETE and TIGUE and lower in NGOUNDJA. However, no significant difference (p >0.05) was observed between sites.
Reproductive characteristics of C .goliath
- Egg diameter
(Table 2) summarises the diameter of C. goliath eggs at the different sites as a function of incubation time.
|
Sites |
Incubation time (j) |
||||
|
1 |
2 |
3 |
4 |
5 |
|
|
GOUNDJA (n=27) |
6.09 ± 0.56ab |
6.13 ± 0.41 ab |
6.15 ± 0.37 ab |
6.43 ± 0.19 ab |
6.93 ± 0.26 ab |
|
BETE (n=10) |
5.80 ± 0.50 ab |
5.93 ± 0.51 ab |
6.20 ± 0.41 ab |
6.23 ± 0.17 ab |
6.93 ± 0.37 ab |
|
TIGUE (n= 7) |
6.18 ± 0.24 ab |
6.33 ± 0.62 ab |
6.35 ± 0.56 ab |
6.36 ± 0.58 ab |
6.37 ± 0.48 ab |
Table 2: Changes in egg diameter (mm) in the Sites studied as a function of incubation time.
(a): in the same row, the numbers with the same letter are not significantly (P>0.5) different
(b): in the same column, numbers with the same letter are not significantly (P>0.5) different.
Oocyte diameter increased with incubation time at all sites with higher values on day 5 of incubation and lower values on day 1. Taking site into account, oocyte diameter was higher in TIGGUE and NGOUNDJA and lower in BETE. However, no significant difference (p >0.05) was observed with incubation time or between sites.
- Fecundity
(Figure 9) illustrates fecundity in C. goliath according to egg laying site.
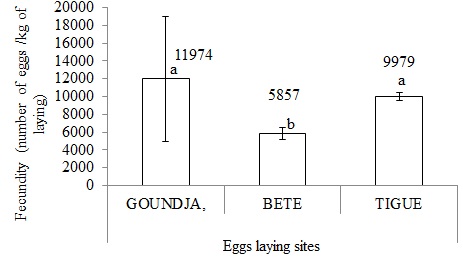 Figure 9: Fecundity in C. goliath according to egg laying site.
Figure 9: Fecundity in C. goliath according to egg laying site.
This shows that fertility was significantly (p < 0.05) higher at GOUNDJA and TIGUE, and lower at BETE.
- Fertilisation rate
The (Figure 10) illustrates the fertilisation rate in C.goliath according to the oviposition sites.
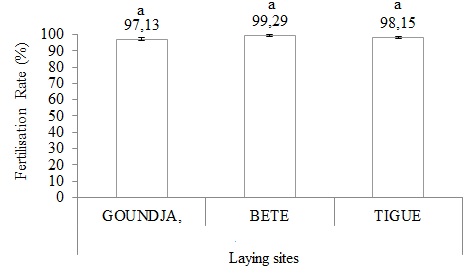 Figure 10: Fertilisation rate variation according to laying sites.
Figure 10: Fertilisation rate variation according to laying sites.
The fertilisation rate was very high and comparable between oviposition sites.
- Colour and shape of Conroua goliath eggs during incubation
During incubation of Conroua goliath eggs in the wild, observation over time revealed variations in egg colour and shape (Table 3).
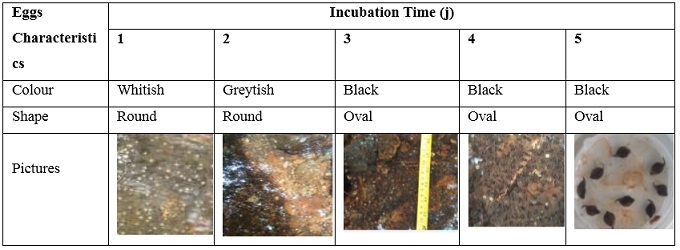 Table 3: Variation in egg colour and shape as a function of incubation time.
Table 3: Variation in egg colour and shape as a function of incubation time.
The colour of the eggs varied during incubation from whitish on day 1 to greyish on day 2 and black on days 3, 4 and 5. Similarly, the shape of the eggs varied during incubation from round on days 1 and 2 to oval on days 3, 4 and 5.
- Incubation time
The incubation period for Conroua goliath eggs was comparable regardless of the oviposition sites. The average incubation period was 5 days.
- Hatching rate
The hatching rate is shown in (Figure 11).
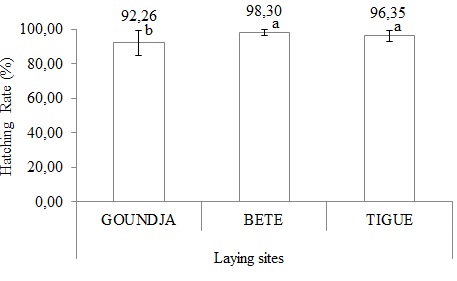 Figure 11: Hatching rate in function of laying sites.
Figure 11: Hatching rate in function of laying sites.
It can be seen that the hatching rate was significantly (p < 0.05) higher at BETE and TIGUE and lower at GOUNDJA.
- Rate of deformed larvae
(Figure 12) illustrates the rate of deformed C. goliath larvae after hatching according to the sites.
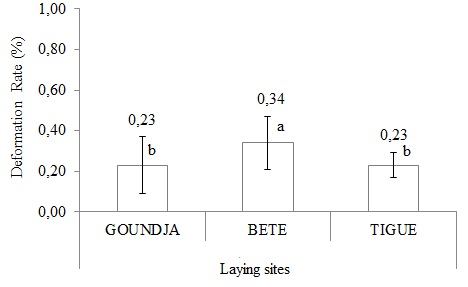 Figure 12: Rate of deformed larvae by watercourse.
Figure 12: Rate of deformed larvae by watercourse.
The average rate of deformed larvae was low and comparable between spawning sites.
- Average weight and total length of C. goliath larvae at hatching
The average weight and total length of C. goliath larvae at hatching are summarised in (Table 4).
|
Growth Characteristics |
Laying Sites |
||
|
GOUNDJA |
BETE |
TIGUE |
|
|
Weight (mg) |
90,00 ± 0,50a |
89,00 ± 0,48a |
91,01 ± 0,36a |
|
Total Length (mm) |
6,92 ± 0,25a |
6,90 ± 0,15a |
6,45 ± 0,18a |
|
Growth Characteristics |
Laying Sites |
||
|
GOUNDJA |
BETE |
TIGUE |
|
|
Weight (mg) |
90,00 ± 0,50a |
89,00 ± 0,48a |
91,01 ± 0,36a |
|
Total Length (mm) |
6,92 ± 0,25a |
6,90 ± 0,15a |
6,45 ± 0,18a |
Table 4: Variation in mean weight and total length of C.goliath larvae at hatchingin relation to oviposition sites.
The average weight and average total length were comparable at all the study sites.
Discussion
Observation of the reproduction of goliath frogs (Conroua goliath) in the wild has shown that they reproduce in fast-flowing streams overflowing with stones and sandy bottoms in the dry season between November and April. This result corroborates Amiet's (2004) description of the habitat in which the same species lives. With regard to the reproduction period, this result is the opposite of studies carried out on the reproductive biology of the African catfish Euchilichthys guentheri, which showed that they reproduce at the beginning of the dry season [11]. During the C goliath spawning period, the breeders build nests on the side of the watercourse in areas that are difficult for predators to access in order to secure their eggs. The choice of this period for breeding could be justified by the considerable reduction in flooding, which would make it easier for C. goliath to lay their eggs in a single layer at the bottom of the nest, where they settle on clean sand and gravel. This observation corroborates that made on the same species in the Mpoula stream in 2019 [13].
The physicochemical characteristics of the water in the nests during the C. goliath breeding period varied very little. During this period the highest mean temperature was 28.47 ± 0.36oC in the nests in the Goundja stream in February 2021 and the lowest mean was 28.22 ± 0.15oC in the nests in the Tigue stream in March 2021. The temperature variation was less than 1oC. This temperature is lower than that obtained for Rana lessonae and Rana esculenta [12]. However, these values are closer to those obtained by (Longhurst, 1968, Boyd 1979, Ceda 1997) in a tropical environment on the study of Chrysichthys nigrodigitatus where the temperature range varied between 250C and 320C. The pH of the nests varied very little, with the highest mean of 6.75 ± 0.07 in April 2021 in the nests of the Tigue stream and the lowest mean of 6.48 ± 05 in April 2022 in the nests of the Goundja stream. These values are clearly lower than those observed in the living environment of Rana lessonae, which places the pH range during reproduction between 7 and 8 [12]. The highest average dissolved oxygen level was 18.7 ± 0.31 mg/l obtained in February in the nests of the Goundja stream in 2022 and the lowest average 15.77 ± 0.15 mg/l observed in the nests of the Goundja stream in March 2022. These values are much higher than those obtained from the study of the physicochemical characteristics of the environments of fishery resources, whose values varied between 3.77 ± 0.9mg/l and 7.39 ± 1.2mg/l [8]. Water conductivity at the study sites varied very little. The highest average 44.07 ± 0.05 µS/cm was observed in the nests of the BETE watercourse in April 2021 and the lowest average 43.96 ± 0.17µS/cm was observed in March 2021.The salinity obtained in the 3 sites was very high. The highest average of 26 ± 0.10 ppm was observed in April 2022 in the Tigue stream and the lowest average of 25 ± 00 ppm was observed in March and April in the nests of the BETE stream and in February and March in the nests of the Tigue stream in February and March 2021. These values are much higher than those recorded by Adekunle et al (2009), who recorded a salinity of between 3.40 ± 0.5ppm and 3.69 ± 0.3 ppm [14].
The distribution of goliath frog nests by study site yielded a highest average frequency of 61.36 ± 1.50% in the Goundja stream and a lowest 16 ± 0.1% in the Tigue stream. The more pronounced human activity is in the frogs' habitat, the less the area is chosen by C. goliath for reproduction. This observation corroborates that made on the amphibian population, which links their decline to fragmentation [2].
The monthly distribution of Conroua goliath nests varied greatly. The highest mean nest frequency of C. goliath was 52 ± 0.01% in March and the lowest mean nest frequency was 12 ± 0.3% in April. The C. goliath egg-laying peak was reached between February and March. In April, the drought is very pronounced, causing rivers to dry up and the nests containing incubating eggs not to be replenished.
All of the C. goliath nests inventoried at the 3 sites had a rectangular quadrilateral shape with the water inlet and outlet directed widthways. The shape observed in the case of this study corroborates the description of Type 1 and 2 C. goliath nests made by Marvin Schäfer et al (2019) in the Mpoula stream on nests of the same species. However, the Type 3 nests (circular shape) described by the same authors were not observed in this study. The surface area of the nests varied according to the sites, with the highest average being 0.30 ± 0.17 m2 in the GOUNDJA stream and the lowest 0.13 ± 0.05 m2 in the nests in the BETE stream. The surface area of the C. goliath nest varied inversely with the slope along the watercourse. The height of the water above the nests was comparable with the highest average of 5.5 ± 0.85 cm in nests in the Goundja stream and the lowest average of 5 ± 0.28 cm in nests in the Tigue stream. These observations are lower than those recorded by Marvin Schäfer et al in 2019 in the Mpoula stream for the same species, where the mean value was 9.1 ± 2.1 cm [13]. The height of water above goliath frog nests is thought to be a determining factor in the development of incubating eggs. Conroua goliaths protect their nests from predators while the eggs are incubating. If, during incubation, the reproductives are captured by predators, the nest is described as an ‘orphan nest’, prey to many harmful factors. This observation corroborates the observation made on the reproductive strategies of green frogs by William E. Duellman (2019). Water circulation speed in Conroua goliath nests varied very little. The highest average was 0.04 ± 0.00 m/s observed in the BETE et TIGUE stream and the lowest average 0.037 ± 0.00 m/s in the Goundja stream. The reasoning behind the speed of the water in the nests would be to clean the physical particles clinging to the eggs and also to permanently oxygenate the incubating eggs.
The diameter of the eggs did not vary during incubation. The highest average observed was 6.18 ± 0.24 mm on day 5 in the Tigue stream and the lowest average was 5.8 ± 0.50 mm in the Bete stream on day 2. The mean diameter of C. goliath eggs remained constant during incubation and was well above that of Rana esculenta recorded by William E. Duellman in 1992, which had an average diameter of 5 mm. This difference may be due to the gigantic size of C. goliaths, which are much larger than other red-legged frogs.
The average relative fecundity varied greatly with a high average of 11974 ± 7061 eggs/kg of spawning observed in the Goundja watercourse and a low average of 5857 ± 671 eggs/kg of spawning in the Bete watercourse. These values are close to those obtained by Lalèyè et al in 1995 on the species Crysichthys aureus and C. nigrodigitatus in Lake Nokoué which had obtained a value of between 18,000 and 11,000 eggs/kg respectively. The fecundity rate of C. goliath eggs in the sites varied little. The highest average fecundity rate was 99.49 ± 0.22% in the Bete stream and the lowest 98.35 ± 0.79% in the Tigue stream.
The colour and shape of the eggs varied during incubation. At the time of laying, the eggs were whitish in colour on day 1, at which time the egg showed axial polarisation with the animal hemisphere pigmented white and the plant hemisphere clear. A whiter annular band can be seen at the equatorial level. The fertilised egg moves towards the perivitelline space. On day 2, the egg takes on a greyish colouration which marks the cleavage into 2 similar blastomere cells and then into 4 cells of the same size. On day 3, the embryo takes on a black colour and is called a blastula. It has several thousand cells and is subdivided into 3 differentiated parts. The ectoderm, which consists of the epidermis and the ventral neuroderm, all located in the animal hemisphere, and the endoderm, which consists of the digestive tract located in the plant hemisphere. The mesoderm, made up of dorsal bone and muscle tissue, develops in the equatorial zone. All these differentiations take place on days 3-4-5. The shape of the eggs varied from round from day 1 to 2 and oval on days 3-4-5. Frog differentiation is the result of the interaction between genetics, non-genetic parental effect and environmental factors that guarantee the phenotype (Mitchell-Olds et al, 2007).
The incubation period of Conroua goliath eggs was comparable independently of watercourse. The average duration was 5 ± 1 days. This result corroborates that obtained by Hickman R. L. (2002) who recorded an incubation period for frogs of 5 days.
The hatching rate of C. goliath eggs was comparable in the 3 sites. The average hatching rate was highest in the Béte stream at 98.30 ± 1.87% and lowest at 89.25 ± 7.23% in the Goundja stream. This hatching rate is close to that obtained by Gudernatsch (1912) when thyroid hormone was identified as an inducer of metamorphosis in frogs.
The rate of deformed larvae was comparable in the 3 sites with the highest average rate 0.34 ± 0.13% in the Bete watercourse and the lowest average rate 0.23 ± 0.26% in the Goundja watercourse. The higher larvae rate is justified by the high impact of human activities in the Bete stream, which leads to eutrophication of the water and therefore asphyxiation of incubating eggs or inhibition of their development. This observation corroborates that of Blaustein and Kiesecker [2].
The average weight of the larvae on the first day of hatching did not vary. The highest average weight of 91.01 ± 0.36 mg was obtained in the Tigue watercourse and the lowest average weight of 89.00 ± 0.48 mg was obtained in the BETE watercourse. The average total length (TL) of Conroua goliath larvae varied very little with a high value of 6.92 ± 0.25 mm recorded in the Goundja stream and the lowest average of 6.45 ± 0.18 mm recorded in the Tigue stream. This information is similar to the observations made on red-legged frogs by William E. Duellman (2019) which recorded an average larval size of 5 mm.
Acknowledgements
Our thanks go to KANGUE Philips and NGWA Nkoullou, both experienced fishermen/hunters of Goliath frogs. For their help in identifying goliath frog nests, breeding sites and periods.
References
- Denver RJ (2017) Endocrinology of Complex Life Cycles: Amphibians. In Hormones, Brain, and Behavior–Tome 2. Academic Press of Oxford: 145-168.
- Adekunle A, Simeon O (2008) Fish Resources And Physico-chemical parameters of Lagoon In Ogun Waterside local Government Area, Ogun State, Nigeria. Journal of Environmental Extension 7: 56-62.
- Blaustein, Kiesecker (2002) Complexity in conservation: Lessons from the global decline of amphibian populations. Ecology Letters 5: 597-608.
- Bertrand S, Laudet V (2001) La métamorphose des amphibiens: Un modèle prometteur pour étudier les protéases de la matrice. Médecine/Sciences 17: 1195-1200.
- Bethany J (2009) Cranial Osteogenesis and Suture Morphology in Xenopus laevis: A Unique Model System for Studying Craniofacial Developpement. PLoS ONE 4: 12.
- Gudernatsch JF (1912) The influence of specific organs given as food on growth and differentiation. Feeding Experiments on Tadpoles : 457-484.
- Heusser S (2021) Dans l’intimité des Grenouilles[en ligne]. Codex virtualis Disponibilité et accè.
- Igou A (2009) Fish resources and physico-chemical parameters of lagoon in ogun waterside local government area, ogun state, Nigeria.
- Jamshed RT (1998) Amphibian metamorphosis as a model for studying the developmental actions of thyroid hormone. Cell Research 8: 259-272.
- Amiet JL (2006) Cycles annuels d’activité vocale chez les Amphibiens Anoures de la région de Yaoundé (Cameroun). Revue d’Écologie 61: 271-302.
- Tembeni JM (2014) Biologie de la reproduction d’un poisson chat Africain Euchilichthys guentheri (Schilthuis, 1891) (Mochokidae, Siluriformes) au Pool Malebo, Fleuve Congo (République Démocratique du Congo). Revue TROPICULTURA 32: 129-137.
- Jerôme M (1997) Etat des populations de "grenouilles vertes" Rana lessonae, Rana kl. esculenta du Bois de Finges (Salquenen, VS) 117: 13-22.
- Shäfera M, AL (2019) Goliath frogs build nests for spawning–the reason for their gigantism? JOURNAL OF NATURAL HISTORY 53: 21-22.
- Pattier JY (1991) Croissance et développement des animaux. Ellipses 127.
- Tétry A (2021) Métamorphose. In Universalis éducation[en ligne]. Encyclopaedia Universalis.
Citation: Charles N, Paul Z, Melvin M, Ebenezer NTI, Kukuru MK, et al. (2024) Some Characteristics of Reproduction in the Natural Environment of the African Giant Frog (Conroua Goliath) (Boulenger, 1906) In the Monomodal Forest Zone of Cameroon. J Aquac Fisheries 8: 088.
Copyright: © 2024 Nguessu Charles, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

