
Therapeutic Effects of Zea Mays (Corn) Husk Aqueous Extracts on the Enzymes, Bilirubin and Condition Factor of Pseudomonas aeruginosa Infected Clarias garipinus
*Corresponding Author(s):
Ukwe IOKDepartment Of Fisheries Sand Aquatic Environment, Rivers State University, Nkpolu-Oroworukwuo, Port Harcourt, Rivers State, Nigeria
Email:oyekuotorisaac@gmail.com
Abstract
Corn (zea mays) husk aqueous extract was assessed for it’s therapeutic effect on enzymes, bilirubin and condition factor of Clarias gariepinus infected with Pseudomonas aeruginosa. One hundred and twenty (120) C. gariepinus were infected with Pseudomonas aeruginosa and observed for diseases presence. Blood sample was taken before and after diseases presence and the infected fish was distributed into four groups and treated with corn husk aqueous extract at different concentrations (treatments): 0.0ml/l, 10ml/l, 20ml/l and 30ml/l. Blood samples were collected from each group after day 3, 6 and 9 of treatment and taken to the laboratory for enzyme and bilirubin analysis. The following enzymes and bilirubin were analysed: aspartate Amino Trasferasse (AST); Alanin Transferase (ALT); Alkaline Phosphate (ALP); Total Bilirubin (TB) and Conjugated Bilirubin (CB). The values for the enzymes activities and bilirubin were higher after the infection (AI) compared to the values before the infection (BI), depicting distortions in the heamatopetic organs such as kidney, liver etc. But after 3-9 days exposure to the corn husk extracts there was restoration of normal enzyme activities and bilirubin content in the fish blood. The condition factor of the experimental fish after 9 days of treatment with the various concentration of the corn husk extracts shows that the fish exposed to the 10ml/l, 20ml/l and 30ml/l of the extract were in good condition compared to the fish in the control group (0ml/l) as a result of loss of appetite in the control group that resulted to serious weight loss.
Keywords
Bilirubin; Condition factor; Enzymes; Clarias gariepinus; P aeruginosa; Zea mays
Introduction
Aquaculture is the practice of raising aquatic organism within properly partitioned aquatic environment [1]. Fish has been proven to contain high amount of protein including omega-3, omega-6 fatty acids .Omega -3 fatty acid that helps in the treatment of some diseases like cardiovascular diseases, eye disorders, cancers and neurological problems [2].
Aquaculture has attracted many investors due to high level of demand for fish and fih products [3]. Fisheries sector has the ability to create new job opportunities and provide more affordable and healthier diets for future generations [4]. Most part of the world rely on fish for protein [5].
There are often huge lossess in aquaculture as a result of disease presence [6]. Disease often lead to a situation where normal physiological functions are been disrupted in the body, with visible symptoms (pathological symptoms) [7], it is one of the major bottle necks in aquaculture with its negative effects on economic and social developments. Poor understanding or complete lack of knowledge in fish disease and control leads to hudge mortality in aquaculture [8]. Bacterial diseases are responsible for mortalities in both controlled and wild fishes throughout the world [9]. These diseases has caused a lot of damages in aquaculture and therefore needs to be controlled by using drugs such as antibiotics under strict control measures, but it has the disadvantages of drug resistance, environmental pollution, depositing on fish flesh etc [6,10]. Most of the causative microorganisms are naturally occurring pathogens which invade the tissue of a fish exposing them to diseases. [11] observed that harmful microbes, nutritional disorders, poor water quality, environmental pollution etc can lead to disease out break in aquaculture.
Plant and plant products can be administered to the fish either by immersion, injections or oral [12-14]. Strater et al, [15] reported the significance of using plant herb extracts as a treatment method against viral diseases in fish. These herbs have proved their efficacy against pathogenic bacteria such as Aeromonas hydrophila, Pseudomonas aeruginosa, Streptococcus, Staphylococus etc [16,17]. These herbs contains phytochemical properties like: antimicrobial, anti-inflammatory, anthelmintic and anticarcinogenic, that can provide direct or indirect defensive mechanism against pathogens or harmful ailments with no effect on fish or environment [6,18,19] and have been proven to be growth promoters and immune stimulants in aquaculture [6,17,20]. Evaluation of enzymes activities and condition factor have been used as means of evaluating disease presence in fish [19,21].
Clarias gariepinus is the most cultured fish in Nigeria and it has good protein content [22], they exist in the wild, can be cultured in enclosures,and are known for their good growth rate unlike Tilapia [23].
Condition factor is used in fisheries and general fish biology studies to evaluate the relationship between the weight of a fish and its length, with the intention of ascertaining comfortability of the environment [24]. Corn husk is the Protective outer covering of a corn it contain 382g cellulose, 445 g hemicellulose, 66g lignin, 19g protein, and 28g ash per kg of dry matter [25], and it is said to have antimalaria properties [26].
Duru [27] reported that corn husk contains proanthocyannins, Ribalmidine, Naringin, Quinine, Flavan-2ol, Anthocyanine, Phenols, Steroids, Phytates, Oxalates, tannins, Resveratol, etc as phytocohemicals. Several work has been done using plant extracts to treat diseases and to reduce the cost of treatment in aquaculture, but no work to the best of our knowledge has been done using corn husk (Zea mays husk) especially in the treatment of C. gariepinus. This research is geared towards using plants parts such as corn husk that seems undesirable to tackle disease problems in our farms to reduce cost associated with disease.
Materials And Methods
- Location of experiment
The experiment was conducted in the Department of Fisheries and Aquatic Environment, Rivers State University (RSU), Port Harcourt, Rivers State, Nigeria.
- Source of experimental fish
One hundred and twenty (120) sub-adults C. gariepinus were obtained from the Rivers State University Aquaculture centre, Rivers State, Nigeria. Observation were carried out on the fish for two weeks to assess the presence of diseases, during this period the fish was fed with a commercial feed twice daily.
- Source of pathogen
Psuedomonas aeruginosa was gotten from the Microbiology Departmentof the Rivers State University, Npkolu-Oroworukwo, Port Harcourt, Rivers State.
- Preparation of experimental herbs
Zea mays husk aqueous extract was prepared using the method of Ukwe and Vopnu [13]. The zea mays husk was washed thoroughly with clean water. It was boiled using 100g of the zea mays husk per litre of water for 30 minutes, filtered, the filtrate was cooled and stored in the refrigerator for use.
- Experimental design
A complete randomized method (CRD) was used, with four (4) treatments in triplicates.
- Experimental procedure
One hundred and twenty (120) sub-adult Clarias gariepnus were infected via intraperitoneal injection with 1.5ml of 105 cfu/ml over night cultured pseudomonas aeruginosa using 2ml injection syringe and 21 guage hypodermic needle, and were observed for disease presence. After disease presence, (lost of appitite and minor alcerations) they were distributed into four (4) groups of ten (10) in triplicate, and were treated via immersion using zea mays husk aqueous extracts at 0ml/L, 10ml/L, 20ml/L and 30ml/L. The treatment/water was replaced every 48 hours.
Blood samples were collected before and after disease occurrence, and after day 3, 6 and 9 of treatment and were taken to the laboratory for analysis to determine the enzymes activities and the bilirubin content. The weight and length were taken before infection and after nine days treatment to determine the condition factor of the various fish.
- Blood extraction
The C. gariepinus was blind folded with a thick cloth to attain calmness, and blood was extracted via kidney puncture using 5ml injection syringe
- Enzymes and bilirubin analysis
The collected blood samples were collected and transferred into LITHUM HEPARIN tube and taken to the laboratory for biochemical analysis. The blood were assayed for aspartate amino transferase (AST), alanine amonitransferase (ALT), alkaline phosphate (ALP), total bilirubin (TB) and conjugated bilirubin (CB). This was done by the use of “Evolution 3000 machine” an auto-analyzer, the screen master model, manufactured by Biochemical system, China. It was used according to manufacturers instructions.
- Condition factor was calculated as:
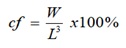 [5]
[5]
W = Weight in grams
L = Length in cm
- Physio-chemical parameters
The physio-chemical parameters (pH, Temperature, DO) were assessed using pH meter for pH, thermometer for temperature, and DO meter for dissolved oxygen.
- Statistical analysis
The data was analysed using SPSS statistics software 17.0 for windows, a one-way analysis of variance was used to determine if there was difference in the variables among treatments. Turkeys multiple comparison test was used to compare the means of the treatment [28]
Results
- Enzymes and bilirubin activities in the plasma of infected clarias gariepinus treated with zea mays husk aqueous extracts
The values of enzymes and bilirubin activities in plasma biochemistry of the experimental C. gariepinus before and after exposure to P.aeruginosa are presented in (Table 1) The results indicated that the values of Aspartate aminotranferase (AST), Alanine transaminase (ALT), Alkaline phosphate (ALP), Total Bilirubin (TB) and Conjugated Bilirubin (CB) in the experimental fish were significantly higher (P>0.05) after infection with the pathogen P.aeruginosa compared to the values before infection.
|
Period |
Enzymes/Bilirubin Parameters |
||||
|
AST (IU/L) |
ALT (IU/L) |
ALP (IU/L) |
TB (Umol/L) |
CB (Umol/L) |
|
|
|
|
|
|
|
|
|
Before infection (BI) |
29.67±2.51a |
15.33±0.91 a |
22.33±5.02 a |
4.67±0.76a |
3.10±0.75 a |
|
After Infection (AI) |
39.33±1.52b |
21.13±1.32 b |
30.67±0.02 b |
8.23±0.64 b |
5.40±0.88 b |
Table 1: Enzymes and Bilirubin activities in the Plasma of the experimental Clarias gariepinus before and after exposure to P. aeruginosa.
Means within the same column with different superscripts are significantly different (P < 0.05)
Key: AST – Aspartate aminotransferase, ALT – Alanine transaminase, ALP – Alkaline phosphatase, TB – Total Bilirubin, CB – Conjugated Bilirubin
The enzymes activities and bilirubin values in plasma biochemistry of infected C. gariepinus after three (3) days of treatment with the zea mays husk aqueous extracts are presented in (Table 2). The activities of all enzymes and bilirubin which include AST, ALT, ALP, TB and CB in the treated fish reduced significantly (P<0.05) with increasing concentrations of the zea mays husk aqueous extracts. However, the values of all the parameters between 10.00 and 30.00 ml of the extracts were within the same range with no significant difference except in AST and CB where higher values of 32.67± 10.21 and 4.27 ± 1.96 were respectively recorded in 10.00 ml of the extracts but the values were significantly higher in all the parameters in the 0.0ml extract except ALP (Table 2).
|
Treatments (ML/L) |
Enzymes/Bilirubin Parameters |
||||
|
|
AST (U/L) |
ALT (U/L) |
ALP (U/L) |
TB (UMOL/L) |
|
|
0.00
|
36.67±3.51 b |
20.07±2.49 b |
28.33±2.08 a |
6.27±1.95 b |
4.67±1.87 b |
|
10.00 |
32.67±10.21 b |
17.83±2.27 a
|
28.00±1.00 a |
5.50±2.33 a |
4.27±1.96 b |
|
20.00 |
29.33±6.65 a |
17.10±2.67 a |
27.00±4.00 a |
5.30±1.90 a |
3.76±1.29a
|
|
30.00 |
20.33±5.68 a |
15.49±0.53 a |
26.33±2.52a |
5.06±1.41a |
3.70±1.34 a |
Table 2: Enzymes and Bilirubin activities in the Plasma of the infected Clarias gariepinus after Three (3) days treatment with the corn husk aqueous extract.
Means within the same column with different superscripts are significantly different (P < 0.05)
Key: AST – Aspartate aminotransferase, ALT – Alanine transaminase, ALP – Alkaline phosphatase, TB – Total Bilirubin, CB – Conjugated Bilirubin
After Six (6) days of treating the infected C.gariepinus with the zea mays husk aqueous extracts (Table 3), the values of the analysed parameters under consideration (AST, ALT, ALP, TB and CB) in the treated fish were reduced but with no significant difference in the 20ml and 30ml/liters compare to the values in 0ml (control) and 10ml/litres which were higher, and the reduction was dose dependant.
|
Treatments (Ml/L) |
Enzymes/ Bilirubin Parameters |
|||||
|
AST (U/L) |
ALT (U/L) |
ALP (U/L) |
TB (UMOL/L) |
CB (UMOL/L) |
|
|
|
|
|
|
|
|
|
|
|
0.00
|
39.33±5.51 b |
20.50±2.69 b |
23.33±4.16 b |
7.20±1.25 b |
5.10±0.87 b |
|
|
10.00 |
35.33±3.51 b |
19.61±3.82 b |
21.00±4.00b |
6.03±1.22 b |
4.40±0.36 b
|
|
|
20.00 |
30.67±4.04 a |
16.77±1.17 a |
20.67±3.21 a |
5.93±0.37a |
3.87±0.40 a |
|
|
30.00 |
29.67±1.52 a |
15.47±2.75 a |
19.67±1.52 a |
5.83±0.41 a |
3.30±0.62 a
|
|
Table 3: Enzymes and Bilirubin activities in the Plasma of the infected Clarias gariepinus after Six (6) days treatment with the corn husk aqueous extract.
Means within the same column with different superscripts are significantly different (P < 0.05)
Key: AST – Aspartate aminotransferase, ALT – Alanine transaminase, ALP – Alkaline phosphatase, TB – Total Bilirubin, CB – Conjugated Bilirubin
After Nine (9) days of treatment of infected C.gariepinus with the zea mays husk aqueous extracts (Table 4.4). The AST was significantly lower in the 30ml/L extract (28.00+5.56), but higher and significantly the same in the rest treatments. It was significantly higher in the control (0.0ml/L) for the ALT, but lower and significantly the same in the rest, while the ALP and TB were significantly the same across the treatments. The CB was significantly higher in the 0.0ml and 10ml/L, but lower and the same in the 20ml and 30ml/L treatments.
|
Treatments (Ml/L) |
Enzymes/ Bilirubin Parameters |
||||
|
AST (V/L) |
ALT (U/L) |
ALP (U/L) |
TB (UMOL/L) |
CB (UMOL/L) |
|
|
|
|
|
|
|
|
|
0.00
|
32.00.±2.00 b |
20.50±2.69b |
27.33±2.52a |
5.80±0.36 a |
5.10±0.52 b |
|
10.00 |
31.67±3.21 b |
17.61±3.82 a |
27.00±5.19 a
|
5.77±0.21 a |
4.03±0.65 b |
|
20.00 |
30.00±3.00 b |
16.77±1.17 a |
24.67±6.50 a |
5.76±1.37 a
|
3.96±0.86 a |
|
30.00 |
28.00±5.56 a |
15.47±2.75 a |
22.67±8.02a |
5.63±0.47a |
3.83±0.40 a |
Table 4: Enzymes and Bilirubin activities in the Plasma of the infected Clarias gariepinus after Nine (9) days treatment with the corn husk aqueous extract.
Means within the same column with different superscripts are significantly different (P < 0.05)
Key: AST – Aspartate aminotransferase, ALT – Alanine transaminase, ALP – Alkaline phosphatase, TB – Total Bilirubin, CB – Conjugated Bilirubin
- Condition factor
The condition factor after infection with the pathogen and treated with the zea mays husk extracts are shown in (Table 5), they were higher and similar in the fish treated with 10ml (1.01+0.10) 20ml (1.00+0.81) and 30ml/L (1.02+0.09) zea mays husk extracts but significantly lower in the control (fish treated with 0.0ml/L of the extract), (0.78+0.55). While the length and the weight of the fish were within the same range with no significant difference (P>0.05).
|
Parameters |
Concentrations of Corn Husk Aqueous Extract (ml/L) |
|||
|
0.00 |
10.00 |
20.00 |
30.00 |
|
|
|
|
|
|
|
|
Weight (g) |
263.33±20.20a |
295.00±5.00 b |
323.33±49.07 c |
330.00±36.05 c |
|
Length (cm) |
32.33±0.57 a |
30.87±0.92 a |
32.80±2.03 a |
31.83±0.32 a |
|
C F |
0.78±0.55 a |
1.01±0.10 b |
1.00±0.81 b |
1.02±0.09 b |
Table 5: Condition factor of the experimental Clarias gariepinus after infection with P. aeruginosa and treated with corn husk aqueous extracted for nine (9) days.
Means within the same row with different superscripts are significantly different (P < 0.05)
Key: C F –Condition Factor
- Comparative values of enzymes and bilirubin activities in the plasma of infected c.gariepinus treated with zea mays husk aqueous extracts within the period of the experiment
Comparative values of AST activities in the plasma of infected C.gariepinus treated with zea mays husk aqueous extracts for nine days is shown in (Figure 1). The results indicated that the values of AST after infection with P. aeruginosa was clearly elevated compared to the values before infection. But after nine days treatment, values of the AST reduced in the treated groups throughout the period of treatment compared to the untreated group.
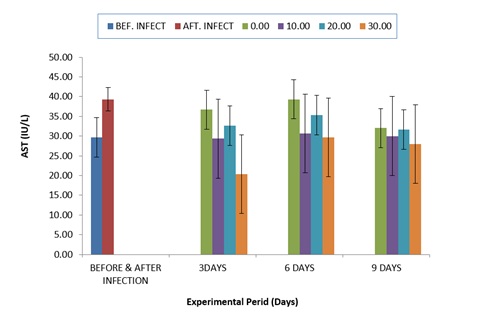 Figure 1: Comparative Values of AST in the plasma of infected C.gariepinus and treated with corn husk aqueous extracts.
Figure 1: Comparative Values of AST in the plasma of infected C.gariepinus and treated with corn husk aqueous extracts.
The comparative value of the ALT is shown in (Figure 2). The value was significantly higher after infection. But after nine days of treatment, it was reduced in the treated group throughout the period of treatment compared to the untreated group (0.0ml), and it was dose dependant after three days treatment.
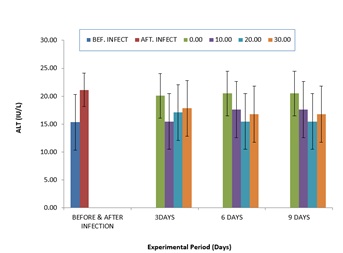 Figure 2: Comparative Values of ALT in the plasma of infected C.gariepinus and treated with corn husk aqueous extracts.
Figure 2: Comparative Values of ALT in the plasma of infected C.gariepinus and treated with corn husk aqueous extracts.
The comparative values of ALP activities in the plasma of infected C gariepinus and treated with zea mays husk aqueous extracts for nine days is shown in (Figure 3). The values of ALP were elevated from 22.33±5.02 to 30.67±0.02 after infection with P. aeruginosa. The values of ALP were progressively reduced after treatment with aqueous extracts of zea mays husk which was more noticeable at six days of treatment. Comparatively, the values of TB (Figure 4.4) were elevated after infection, but after treatment with zea mays husk aqueous extracts, the values of TB were reduced when compared to the after infection values. However, higher values were observed in the control at day 3 and 6, respectively (Figure 4). The comparative values for the CB had the same trend with the value of TB throughout the period of experiment (Figure 5).
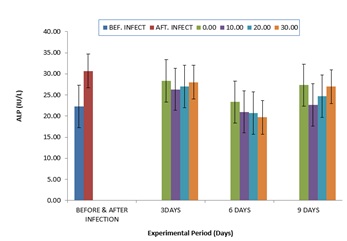 Figure 3: Comparative Values of ALP in the plasma of infected C.gariepinus and treated with corn husk aqueous extracts.
Figure 3: Comparative Values of ALP in the plasma of infected C.gariepinus and treated with corn husk aqueous extracts.
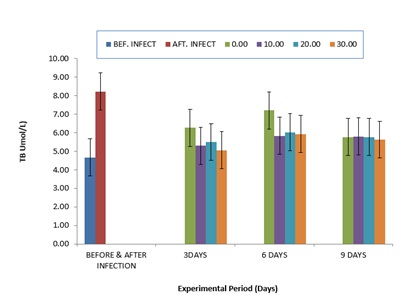 Figure 4: Comparative Values of Total Biluribin (TB) in the plasma of infected C.gariepinus and treated with corn husk aqueous extracts.
Figure 4: Comparative Values of Total Biluribin (TB) in the plasma of infected C.gariepinus and treated with corn husk aqueous extracts.
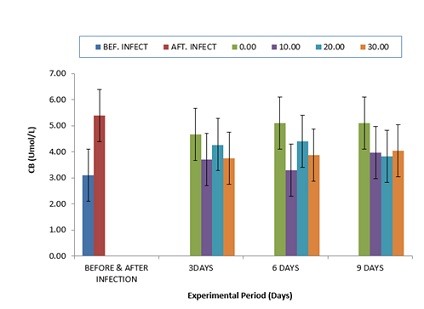 Figure 5: Comparative Values of Conjugated bilirubin (CB) in the plasma of infected C.gariepinus and treated with corn husk aqueous extracts.
Figure 5: Comparative Values of Conjugated bilirubin (CB) in the plasma of infected C.gariepinus and treated with corn husk aqueous extracts.
Discussion
- Effects of zea mays husk extract on the Enzymes activities in the plasma of P.aeruginosa infected C.gariepinus
Biochemical properties such as aspartate transaminase (AST), alanine transaminase (ALT) and alkaline phosphate (ALP) are biomarkers used to access the wellness of fish body organs such as kidney, liver and other hematopoietic organs [13-29]. Ghelichpour et al [30] described the AST and ALT as the most important aminotransferase that are non-functional enzymes and exist mainly in the liver and kidney. Ukwe and Oladapo [31] postulated that increase in these enzymes are associated with diseases, when P.aeruginosa and A.hydrophila were intermuscularly injected into C.gariepinus, and further stated that the level of post infection increase in these enzymes depend on the type and virulence of the pathogen or disease agent. [32] also reported an increase in ALP when fasciolaisis gigantic was experimentally infected to Balami sheep, and argued that the post infection elevation maybe as a result of distortions in the bile duct. The analysis of these enzymes (AST, ALP and ALT) are normal laboratory test to detect abnormalities in farmed animals including fish [19,33], and alterations in these enzymes in fish plasma as a result of therapeutic drug/herbs applications have been reported [13,34]. In this study, there were significant increase in the AST, ALT and ALP after forty-eight (48) hours infection with P.aeruginosa. Similar observations were reported by Ukwe and Etire [19] when C. gariepinus was intraperetoneally injected with P. aeruginosa and exposed to Persea americana aqueous leave extract and Nnabuchi et al [35] also reported increase in these enzymes when the effects of parasites in the biochemical and the haematological indices of some clariid catfish from Anambra river in Nigeria was evaluated. Significant increase in these enzymes were observed in the P. aeruginosa infected C. gariepnus exposed to the 0.0ml zea may husk aqueous extact (control) all through the period of this study. The significant increase of these enzymes (AST, ALT and ALP) in the plasma of the control after day 3, 6 and 9 is instructive to the fact that the experimental pathogen (P.aeruginosa) had negative effect on the organs (liver, kidney etc..) of the experimental fish exposed to 0.0ml Zea mays husk extracts (control) and could have altered the permeability and strength of the cell organelles to utilize these enzymes [31,35,36] which can negatively affect protein metabolism and cellular damage in the organs. However after day three (3), six (6) and nine (9) of exposure to corn husk aqueous extracts (Figure 4.2-4.4) there were restorations (reduced) in the enzymes activities in the treated groups (10ml, 20ml and 30ml zea mays husk aqueous extracts).This could be as a result of the medicinal phytochemicals in zea mays husk extract [6] which have been reported to be antibacterial with the ability to thicken the cell membranes of the fish internal organs and reduce the potency of the experimental pathogen (P. aeruginosa ) on the fish [19,37]
- Effects of Zea mays husk extracts on Bilirubin Presence in Plasma of P. aeruginosa infected C. gariepinus
According to Hastuti et al [38], the liver outside other functions is involved in the conjugating and secreting of bilirubin which is a product of the break down of red blood cells (haem destruction). This is also supported by Vaglibijaini [39]. High presence of bilirubin in the blood indicates abnormal break down of red blood cells or reduced absorption of bilirubin by the liver, and it is indicated by the presence of yellow pigmentation [40]. Bilirubin is conjugated in the liver, and high presence of conjugated bilirubin in the plasma depicts liver malfunction. Fish affected with high bilirubin presence (hyperbilirubinemia) suffers from insufficient red blood cells which manifest as jaundice (yellow pigmentation) in the liver, body fat, spleen etc [41]. Fish with high presence of bilirubin in their blood increases liver enzymes such as ALT by more than 10% [42]. Both Abbas et al [43], and Tsai and Tang [44] agreed to the fact that high presence of bilirubin in the blood is a product of imbalance process of red blood cell breakdown to bilirubin and the ability of the liver to conjugate it, a process that depicts bile duct injury. In this study, there were significant increase in the total bilirubin (TB) and conjugated bilirubin (CB) after infecting C.gariepinus with P. aeruginosa and the increase continued in the untreated group (0.0ml zea mays husk aqueous extract) after 3, 6 and 9 days of treatment. Similar results were reported by Hastuti et al [38] when total conjugated bilirubin was investigated in jaundice and healthy catfish, and Anagor et al [45] when African catfish was experimentally infected with single and mixed Escherichia coli and Salmonella gallinarium. Increased presence of TB and CB after the infection period (Figure. 4.4 and 4.5) depicts high breakdown of red blood cells or inability of the liver to conjugate the bilirubin i.e liver dysfunction [45]. However in the infected fish exposed to the various concentrations of the zea mays husk extracts (10ml/l, 20ml/l and 30ml/l). there were TB and CB diminution, revealing restoration to the process of red blood cells destruction and bilirubin removal by the liver. This could be as a result of the antibacterial properties of the phytochemicals contained in the zea mays husk aqueous extract or the boosting of the fish immune system by the phytochemicals to reduce the virulence of the experimental pathogen [19,46].
- Effects of Zea mays husk on the Condition factor of P.aeruginosa infected C.gariepinus
The condition factor is the ratio of the weight of the fish to three times its length express in percentage and it expresses the conduciveness of the fish environment [24].This can be said to be true if the environmental conditions across the experimental treatments are the same. In this study the environmental conditions were the same, and the condition factor was based on the presence of the experimental pathogen in the fish, and concentration of the zea mays husk extracts as express in the weight and length of the experimental fish. The weight of the experimental fish before infection was significantly high compare to the weight after nine days treatment in the untreated group (0.0ml zea mays husk extract ) this is because of the negative pathogenic effects in the infected fish that resulted to loss of appetite [6]. The group treated with 10ml, 20ml, and 30ml of the zea mays husk increased in weight after nine days treatment. This could be as a result of some phytochemicals such as phenols, steroids etc contained in the zea mays husk [19, 27] which may have stimulated or maintained appetitie in the experimental fish [47-49]. The length of Clarias gariepinus increased in both the untreated and treated groups after infection, and the condition factor decreased after infection in the 0.0ml but increased in the rest 10ml, 20ml and 30ml/L of zea mays husk extracts. The increase in the condition factor as a result of continuous weight increase in the treated groups shows that the fish are in good condition.
Conclusion
Zea mays (corn) husk are seen littered all over our communities as wastes. This study have gone a long way to express the usefulness of this waste product. The result of this study reveals that the zea mays husk extracts at the used concentrations has the potency of restoring enzymatic and bilirubin alterations, and the condition factor in Clarias gariepinus infected with bacterial pathogen such as P. aeruginosa, due to loss of appetite resulting to loss of weight. The result also confirms that excess bilirubin in the plasma of the infected fish is a sign of malfunctioning of the kidney and undue destruction of the red blood cells. Zea mays husk aqueous extracts can be used as a therapeutic agent in the practice of aquaculture especially at 10ml and 20ml per liter of water where it performs best. Other Extracting medium should be used to compare results since solvent/means of extraction affects phytochemical content of the plant extracts.
Zea mays husk aqueous extracts should be applied in other aspect of Aquaculture such as growth and flesh quality. Zea mays husk aqueous extracts should be applied in other farm animals for it's medicinal values.
References
- ADCP (2015) Aquaculture planning in Africa. Report of the first Regional Workshop on Planning in Africa. Accra Ghana 2-27.
- Ukwe IOK, Edun OM, Akinrotimi OA (2018) Growth and Microbial Indices of African Catfish (Clarias gariepinus) larva fed formulated and commercial diet. Journal of Fisheries Sciences Committee 12: 001-008.
- Corbould C (2013) Feeding the cities: Is urban agriculture the future of food security. Journal of Agriculture 22: 10-20.
- FAO (2016) Climate change amtications for fisheries and aquaculture summary of the findings of the intergovernmental panel on climate change fifth assessment report by Seggel& C. De Young. FAO Fisheries and Aquaculture cicular1122 Rome.
- James LE, Frank A, Taryn G (2019) Economics of Aqua culture policy and regulaing. Annual Review of Resource Economics 11 : 101-123
- Ukwe IOK, Gabriel UU (2019) Herbs and Herbal Supplements key to productive, Healthy and Eco-Friendly Aqauclture. Delta Aquaculturist 11: 55-67.
- Idowu TO, Ogundaini AO, Adesanya SA, Onawunmi GO, Osungunna MO, et al. (2016) Isolation and Characterization of Chemical Constituents from Chrysophyllumalbidum G. Don-holl. Stem-bark extracts and their Antioxidant and Antibacterial Properties. Afr J Tradit Complement Altern Med 13:182-189.
- Schmidt JW, Agga GW, Bosilevac JM, Brichta-Harhay DM, Shackelford SD, et al. (2015) Haematological, biochemical and clinical sings changes following experimental infection of Streptoccoccus agalactiae in red hybrid tilapia (Oreochnomis sp). Basic Research Journal 4: 289-295.
- Defoirdt T, Sorgeloos P, Bossier P (2011) Alternatives to antibiotics for the control of bacterial disease in aquaculture. Current Opinion Microbiology 14: 251-258.
- Harikrishnan R, Hoseinifar SH, Sun YZ, Zhou Z, Doan HV, et al. (2020) Boosting Immune Function and Disease Bio-Control Through Environment-Friendly and Sustainable Approaches in Finfish Aquaculture: Herbal Therapy Scenarios. Reviews in Fisheries Science & Aquaculture 28: 303-321.
- Kumar JSS, Anantharaja K (2012) Herbalhealth care in aquaculture –the Indian experience. Aquaculture INFOFISH International 12-16.
- Lawal MO, Aderolu AZ, Gafari WA (2021) Effects of Terminalia latapa, chromolaena odorata, and Psidinal guajava leaf Extracts on Growth, Brochemical and Haematology of Clarias gariepinus FUW Trends in Sci and Tech J 6: 327-332.
- Ukwe IOK, Vopnu FB (2021) Diseases Resistance and Enzymatic Changes in Pseudomonus aeruginosa Infected Clarias gariepinus treated with Carica Papaya root extracts. J of Med Care Res and Rev 1079-1089.
- Ukwe IOK, Deekae SN (2022) Phtochemical Assessment of Persea americana powdered leaves and it’s potency in protecting clarias gariepiuepinus against Klebsiella pneumonia. Asain Journal of Fisheries and Aquatic Research 2 : 97-104.
- Stratev, D, Zhelyazkov G, Noundou XS, Krause, RWM (2018) Beneficial Effects of Medical plants in fish diseases. Aquaculture International 26: 289-308.
- Van der WD, Nord CE (2000) Development and persistence of multi-resistance to antibiotics in bacteria; an analysis and a new approach to this urgent problem. International Journal Antimicrobial Agents 16: 191-197.
- Ukwe IOK, Jamabo NA (2020) Effect of dietary mango bark on Clarias gariepinus (Burchells, 1822) infected with Pseudomonas aeruginosa. World J of fish and Marine Sci12: 74-80.
- Velu G, Palanichamy V, Rajan AP (2018) Phytochemical and pharmacological importance of plant secondary metabolites in modern medicine. In Bio-organic Phase in Natural Food 135-156.
- Ukwe IOK, Etire DI (2021) The effects of Perseaamericana leaves on the Enzymes and organosomatic indices of Pseudomonas aerugonosa Infected Clarias gariepinus. Journal of Medical Care Research and Review 4: 1067-1078.
- Zaid A, Isibo P, Aduljehu O, Akinsanya B, Stoilova I, et al. (2020) Effects of herbal mixture (Jedi, Gbewutu and 2005. Antimicrobial and antioxidant activity of Opa-eyin) on the health status of Juveniles Clariasthe polyphenol Mangifera Herb Polonica, 51: 37-44.gariepinus. Egyptian Journal of Aquatic Biology and 35. Yu, J.H., J.J. Han and S.W. Park, 2010. Heamatological Fisheries 24: 31-48.
- Seher D, Suleyman CI (2012) Condition factors of seven cyprinid fish species from Çamligöze Dam Lake on central Anatolia, Turkey. African J of Agri Res 7: 4460-4464 .
- Nelson JS, Grande TC, Wilson MVH (2016) Fishes of the world. Animal Science and Zoology. John Wiley and Sons Inc 15-19.
- Olakunle O (2013) The Growth Performance and Survival of ClariasGariepnus Fry Raised in Homestead Concrete Tanks. Journal of Fisheries and Aquatic Science 8: 233-247.
- Fishbase (2013) Online fish identification sheet.
- Barl B, Bilideris CG, Murray ED, Macgregor AM (1990) Combined chemical and enzymatic treatments of corn husk lignocellulosics. Journal of Sciences of Food and Agriculture 56: 214.
- Okokon JE, Antia BS, Mohanakrishnan D, Sahal D (2017) Antimalarial and antiplasmodial activity of husk extract and fractions of Zea mays. Pharmaceutical Biology 55: 1394-1400.
- Duru, D.C (2019) Mineral and phytochemical evaluation of Zea mays husk. Scientific Africa, 7 : 1-8.
- Wahua, T.A.T (1999) Applied statistics studies. African links books. Aba Nigeria, 365.
- Zaki MS, Sharaf NE, Osfor HM (2007) Effect of vanadium toxicity on biochemical, haematological and clinic-pathological change, in Clariaslazera present in River Nile. American-Europian Journal of Agricultural Environment 2: 741-745.
- Ghelichpour M, Mirghaed AT, Hoseini SM, Nez AP (2020) Plasma antioxidant and hepatic enzymes activity, thyroid hormones alterations and health status of liver tissue in common carp (Cyprinuscarpio) exposed to lufenuron. Aquaculture 516: 734-
- Ukwe IOK, Akinfolarin TT (2019) Alteration in enzyme activities of Clarias gariepinus infected with Aeromonas hydrophila and Psedomonas aeruginosa. Asian Journal of Fisheries and Aquatic Research 4: 1-9.
- Ahmed MI, Ambali AG, Baba SS (2006) Haempathological and biochemical responses of Balami sheep to Experimental Fasciola gigantic infection. Journal of Food Agriculture and Environment 4:71-74.
- Saka WA, Aklige RE, Ishola OS, Ashamu EA, Adeleke GA (2011) Hepototherapeutic effect of Aloe-vera in alcohol-induced hepatic damage. Parkistan Journal of Biological Sciences 14: 742-745.
- Kabir MA (2009) Sublethal Effects of tobacco (Nicotuanatobaccum) leaves dust on enzymatic activities of Heteroclarias (a hybrid of Heterobranchus bidorsalis and Clarias gariepnus). Jordan Journal of Biological sciences 2: 151-158.
- Nnabuchi, UO, Ejikeme, OG, Diduigwu NC, Ncha OS, Onahs SP, (2015) Effects of Parasites on the biochemical and haematological indices of some clarrid (silurifomes) catfish from Anambra, Rivers State, Nigeria
- Adam KM, Iioba KI (2008) Effect of sub lethal concentrations of partland cement powder solution on the amino transferase of the African catfishes (Clarias gariepinus, Burchell, 1822). Acta Zoological Lituanica 18: 50-54.
- Davi G, Patromo C (2007) Platelet Activation and Atherothrombosis. New England Journal of Medicine 357: 2482-2494.
- Hastuti S,Windarto S, (2019) Blood performance of jaundice catfish Clarias gariepinus. AACL Biological flux 12: 480-489.
- Vaghbijiani ST (2008) Function of the liver. In: Drugs and the liver: A guide to drug handling in liver dysfunction. North-Lewis P Pharmaceutical Press London
- Clayton M (2009) Jaundice. In: Liver diseases: an essential guide for nurse and health care professionals. Wiley-Blackwell Publication
- Chinabut S (2002) Jaundice disease in catfish, a case study demonstrating a decline in incidence as a result of research output. FAO Technology 406: 77-80.
- Pratt DS (2010) Liver chemistry and function test. In: Sleisenger and Fordtran’s gastrointestinal and liver disease. Feldma M., Friedma L. S., Brandt L. J., (eds), Saunders Elsevier, Philadelphia, P. A. Journal of Anatomy 2480: 60-71.
- Abbas MW, Shamshad T, Ashraf MA, Javaid R (2016) Jaundice: a basic review. International Journal Research Medical Sciences 4: 1313-1319.
- Tsai M-T, Tarng D-C (2018) Beyond a Measure of Liver Function-Bilirubin Acts as a Potential Cardiovascular Protector in Chronic Kidney Disease Patients. Int J Mol Sci 20: 117.
- Anagor TA, Chah KF, Omeje VO, Anene BM (2019) Haemato-biochemical Parameters in African Catfish Experimentally Infected with Single and Mixed Escherichia coli and Salmonella gallinarium. Journal of Agriculture and Food Technology 9: 7-12.
- Chakraborty P, Ghosh S, Goswami SK, Kabir SN, Chakravarty B, et al. (2013) Altered Trace Mineral Milieu Might Play an Aetiological Role in the Pathogenesis of Polycystic Ovary Syndrome. Biol Trace Elem Res 152: 9-15.
- Reverter M, Bontemps N, Lecchini D, Banaigs B, Sasal P (2014) Use of plant extracts in fish. International Journal of Fisheries 3: 16-20.
- Kumar DB, Singh NR, Bink D, Devashish K (2014) Length-weight relationship of Labeorohita and Labeogonius (Hamilton-Buchanan) from Sone Beel, the biggest wetland of Assam. Indian Journal of Environmental Research and Development
- Lawal MO, Aderolu AZ, Gafari WA (2021) Effects of Terminalia latapa, chromolaena odorata, and Psidinal guajava leaf Extracts on Growth, Brochemical and Haematology of Clarias gariepinus. FUW Trends in Science and Technology Journal 6: 327-332.
Citation: Ukwe IOK, Davies OA, Nwemem GS (2023) Therapeutic Effects of Zea Mays (Corn) Husk Extracts on the Enzymes, Bilirubin and Condition Factor of Pseudomonas aeruginosa Infected Clarias garipinus. J Aquac Fisheries 7: 63.
Copyright: © 2023 Ukwe IOK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

